Biomedical Engineering Reference
In-Depth Information
(a)
(b)
CH
2
OH
CH
2
OH
O
O
O
O
OH
OH
*
*
*
*
NH
NH
n
n
CH
2
CH
2
COOH
COOH
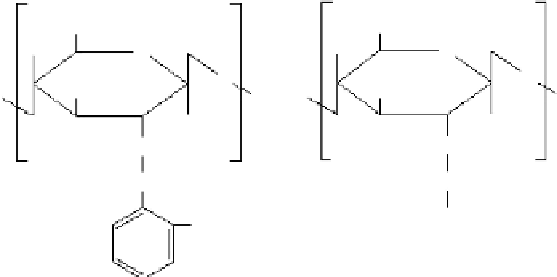
Figure 1.16
(a)
N
-carboxylbenzyl chitosan and (b)
N
-carboxylmethyl chitosan.
to form
N
-hydroxylbenzyl chitosan [76]. The product specifically chelates Cu and Hg. The
product is
N
-carboxylbenzyl chitosan when salicylaldehyde is replaced by phthalaldehy-
dic acid, and the product is
N
-carboxylmethyl when chitosan reacts with glyoxylic acid
[77,78]. The said compounds become insoluble metal chelates after combining with transi-
tion metal ions in water solution, which are easy to separate from water. The compounds
are soluble in acid and alkali solutions and their structures are shown in Figure 1.16.
Glyoxal, glutaraldehyde, and dialdehyde starch are usually used as the cross-linking
agents for chitosan. If the reaction is controlled properly, the expected Schiff base can be
formed. Only one aldehyde group of dialdehyde is used for reacting, which means that the
rest are available for other reactions.
Aminos exist on chains of the chitosan. Muzzarelli and Tanfani [75] claim that
N
-trimethyl chitosan quaternary ammonium salt iodide can be formed from methyl
iodide and chitosan as shown in Figure 1.17.
The specific steps are as follows: drying 5 g of
N
-dimethyl chitosan at 80°C overnight,
adding it to a mixture including 100 mL of anhydrous acetonitrile and 2.5 mL of methyl
iodide, stirring at 35°C continuously in the anhydrous condition for 30 h, and extracting
the product by using ether in a Soxhlet extractor to remove the remaining methyl iodide.
Finally, 6.1 g of the final water-insoluble product is formed.
Considering one residue, the quaternary ammonium salt has a quaternary ammonium
group on C2 of the pyranoid ring of the anhydroglucose unit. All carbon atoms of the
anhydroglucose unit are asymmetric carbon atoms except for C6. The asymmetric carbon
atom may give quaternary ammonium salt special biological activities, for example, selec-
tive bactericidal effect. A phase transfer catalyst with asymmetric carbon atoms may have
some special effects on phase transfer catalysis in asymmetric organic synthesis.
CH
2
OH
CH
2
OH
O
O
O
O
+
OH
OH
n CH
3
l
*
*
*
*
N(CH
3
)
2
N(CH
3
)
3
+
l
-
n
n
Figure 1.17
N
-trimethyl chitosan quaternary ammonium salt iodide.

Search WWH ::

Custom Search