Biomedical Engineering Reference
In-Depth Information
CH
2
OH
CH
2
OH
O
O
O
H
2
O
O
H
CH
3
OH
+
H
2
C
C
OH
*
*
*
*
NH
NH
2
n
n
CHCH
2
CH
2
OH
OH
Figure 1.13
Addition reaction of chitosan and glycidol.
of chitosan forms 6-
O
-cyanoethyl chitosan at 20°C without influencing aminos. When the
reaction temperature reaches 70°C, 30% aminos will be substituted by cyanoethyl.
1.5.4 N-Alkylation
Aminos of chitosan are primary aminos with lone-pair electrons and strong nucleo-
philicity. They are involved in many reactions. N-alkylation is also of great importance
in addition to N-acylation. Each acetamino of chitin is stable because there is only one
hydrogen atom on the nitrogen atom. However, the substitution reaction is still available
in the strong condition.
In the reaction of chitosan and alkyl halide, N-alkylation begins first [71]. The addition
reaction of chitosan and epoxides forms
N
-alkylates and introduces two hydrophilic
hydroxyls at a time. As shown in Figure 1.13, the N-substituted product formed by glycidol
and chitosan is water soluble.
When chitosan reacts in a water solution of excess glycidol, two H atoms on the amino
of chitosan will be substituted to form
N
,
N
-dihydroxyl
n
-butyl chitosan, which is water
soluble.
In neutral medium, chitosan easily reacts with aromatic aldehyde or ketone or aliphatic
aldehyde to form Schiff base. Such a reaction is useful in research and applications of
chitosan. On the one hand, it protects aminos by protective groups that can be easily
removed after the reaction, so that hydroxyls can react freely. On the other hand, Schiff
bases formed by special aldehydes can be used for synthesizing useful
N
-derivatives via
sodium borohydride reduction.
Chitosan Schiff bases are prepared by the following steps [72]: dispersing 1 g of chitosan
powder into methanol, adding 3 mol of aldehyde to the mixture, extracting it by methanol
via a Soxhlet extractor for 4 h, washing with ether, removing residual aldehyde, and drying
by air. Neither aliphatic aldehyde nor aromatic aldehyde can completely change all aminos
of chitosan into Schiff base.
The chitosan Schiff bases can be decomposed by acids to recycle chitosan; for example,
chitosan-salicylaldehyde Schiff base can be decomposed by 5% acetic acid.
Chitosan and formaldehyde hydrate form
N
-hydroxylmethyl chitosan by condensing, as
shown in
Figure 1.14
[73].
The H remaining on the N can further react with one molecule of formaldehyde to form
N
-dihydroxylmethyl derivative, as shown in
Figure 1.15.
The N-monosubstituted product turns into a Schiff base of formaldehyde and chitosan
after one molecule of water is removed.
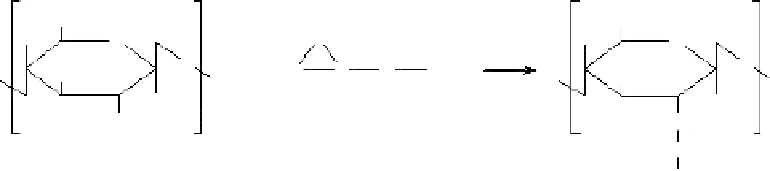
Search WWH ::

Custom Search