Biology Reference
In-Depth Information
4.1.4.2 Photochemical oxidaion of Tyr for cross-linking and labeling
Native CPMV particles display addressable Tyr residues. Structural data
and the study of Tyr-minus mutants demonstrated that two Tyr side chains
located in the S subunit are available for chemical conjugation (Meunier
et al.
, 2004). The Tyr phenol can be oxidized by one electron, allowing
activation and subsequent bioconjugation. Two strategies were explored. In
the first approach, Tyr residues were oxidized by one electron via treatment
with the nickel(II) complex of the tripeptide Gly-Gly-His in the presence
of magnesium monoperoxyphthalate (Ni/GGH/MMPP). In the second
approach, the photochemical action of the tris(bipyridyl)ruthenium(II)
dication was exploited. Both treatments mediated covalent conjugation
of adjacent Tyr side chains and led to effective cross-linking of adjacent
pentameric subunits. Besides cross-linking the Tyr side chain, functional
groups can also be introduced using this type of chemistry. In a proof-of-
concept study, fluorescein has been covalently attached to CPMV Tyr side
chains (Meunier
et al.
, 2004).
4.1.5 Carbohydrate-Selecive Chemistries
Some viruses, such as the archaeal virus
rod-
shaped virus 2 (SIRV2), are glycosylated, allowing chemical modification
using carbohydrate-selective chemistries (Steinmetz
Sulfolobus islandicus
, 2008a) (see also
Section 4.3.5). In order to make use of carbohydrates, the hydroxyls must be
converted into aldehydes. This is typically achieved under mild oxidation
conditions using the reagent sodium
et al.
-periodate. The aldehyde groups
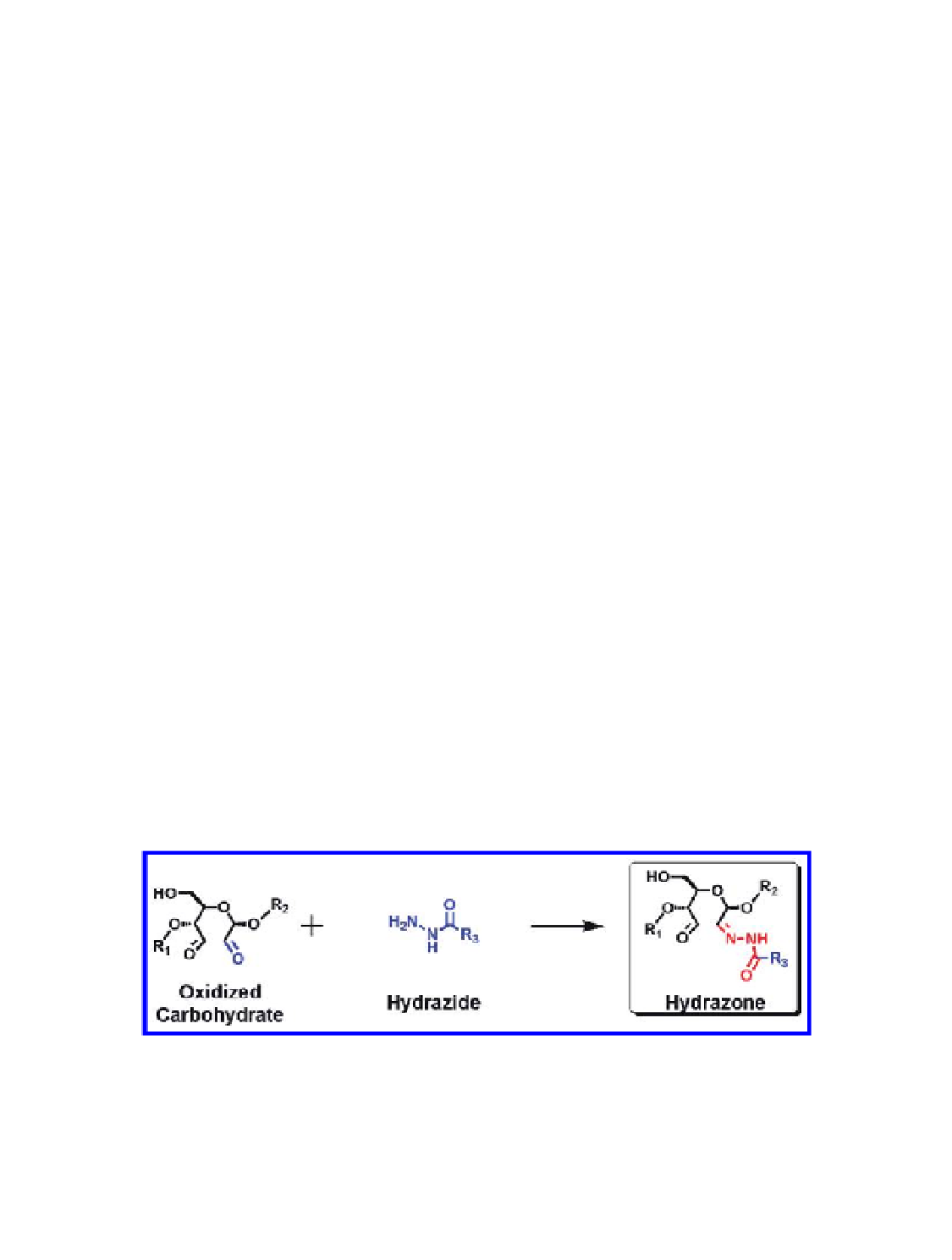
are reactive toward hydrazide conjugates and undergo a facile coupling
reaction that results in the formation of a covalent hydrazone linkage
(Fig. 4.5) (Aslam & Dent, 1999; Hermanson, 1996).
meta
Figure 4.5
Coupling reaction between an oxidized carbohydrate and a hydrazide
derivative. Figure provided by courtesy of Vu Hong (TSRI, La Jolla, CA, USA).

Search WWH ::

Custom Search