Biology Reference
In-Depth Information
4.1.5.1 Chemoselecive glycoconjugaion method
The feasibility of synthesis of
-linked glycoconjugates through site-specific
ligation of 1-glycosyl thiols to proteins has been demonstrated utilizing the
bacteriophage Q
S
b
. The strategy exploits non-natural amino acid incorporation
(recall Section 3.2.1), in this case l-homoallylglycine (l-Hag). Free-radical
glycosylation reaction allows the synthesis of
S
-linked glycoconjugates
(Fig. 4.6) (Floyd
, 2009). This method facilitates the incorporation of
carbohydrates, which could be used for further modification. Alternatively,
modified carbohydrates could be introduced.
et al.
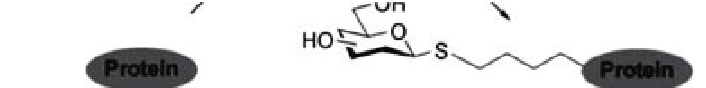
Figure 4.6
-linked glycoconjugates through site-specific ligation of
1-glycosyl thiols to proteins. Reproduced with permission from Floyd, N.,
Vijayakrishnan, B., Koeppe, J. R., and Davis, B. G. (2009) Thiyl glycosylation of olefinic
proteins:
Synthesis of
S
S
-linked glycoconjugate synthesis,
Angew. Chem. Int. Ed. Engl.
,
48
(42),
7798-7802.
4.2 BIO-ORTHOGONAL REACTIONS: CLICK AND OXIME
reACtIonS
There is need for alternative bioconjugation methods, such as the click
reaction (Section 4.2.1) or oxime ligation (Section 4.2.2). Standard coupling
procedures using NHS ester- or maleimide-activated reagents have slow
reaction kinetics, and large excesses of reagents have to be used to facilitate
efficient labeling. Click reaction and oxime ligation are highly efficient
bioconjugation methods that require low concentration and excess of the
reagent or ligand of interest. This is helpful when reagents are scarce or if
solubility in aqueous conditions is a problem. Another advantage is lower
costs, as less material is required.







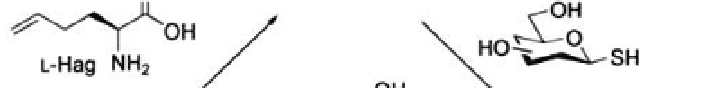

Search WWH ::

Custom Search