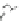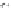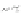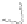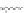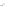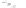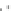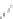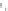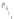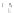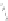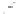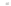Biomedical Engineering Reference
In-Depth Information
a
b
c
N
N
NH
HN
N
N
N
N
CdSe
ZnS
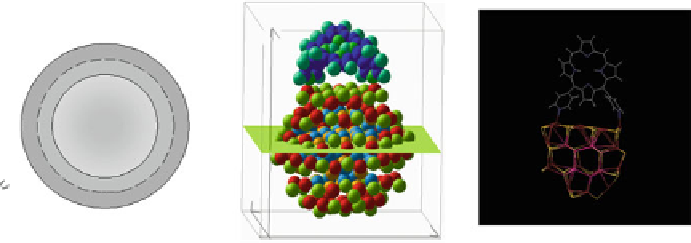
Fig. 4.7
Schematic presentation of “CdSe/ZnS QD-H
2
P” nanoassemblies. (
a
) the scales of CdSe
core (
d
CdSe
=
2.5 nm), ZnS shell, (
m
-Pyr)
4
-H
2
P and TOPO molecules as well as intercenter
distances correspond to relative sizes of the main components of the assemblies [
62
]: the ZnS
shell thickness for QDs was estimated on the basis of one ZnS layer thickness
l
=
0.5 nm;
parameters for conical TOPO molecules
r
bottom
=
0.55 nm,
h
con
=
0.99 nm were used; for (
m
-
Pyr)
4
-H
2
P molecule (see Fig.
4.2
).
r
m
0.75 nm is the radius of a porphyrin molecule with
opposite pyridyl rings having nitrogens in meta-positions,
h
=
10 A is the mean distance between
meta-nitrogens of adjacent pyridyl rings (HyperChem software, release 4.0 geometry optimization
with semiempirical PM3 method). (
b
,
c
) optimized geometry for Cd
33
Se
33
=
(mˆPyr)
2
-H
2
P
(optimization by HyperChem 7.0; simulations by ab initio density functional theory, DFT, with
the VASP code [
115
])
+
assuming an infinitely strong coupling would not result in a dependence of the
quenching on the number of pyridyl rings.
The above presented results lead to the conclusion that in “QD-H
2
P” nanoassem-
blies, H
2
P molecules anchor on the CdSe/ZnS surface in a nearly perpendicular
fashion with two nitrogen lone pair orbitals (at most) forming coordination bonds
with the surface. From geometric arguments, the QD PL weak quenching behavior
observed for H
2
P molecules with opposite pyridyl rings can thus be easily ratio-
nalized because a contact of opposite pyridyl rings to the surface is impossible due
to geometric (steric) reasons in the case of a parallel orientation of the porphyrin
macrocycle with respect to the QD surface. Theoretical simulations (ab initio DFT
with the VASP code [
115
]) have shown also that for the optimized geometry of
QD-H
2
P nanoassemblies, the mutual arrangement of (
m
-Pyr)
4
-H
2
P molecules is
perpendicular relative to QD surface. Considering space-filling molecular entities
for such nanoassemblies, in the case of competitive exchange of TOPO capping
molecules by attaching porphyrin ligands of one H
2
P molecule may replace about
two to three TOPO molecules or, alternatively, fills a free volume corresponding
to two to three TOPO molecules. The overall structural information for QD-H
2
P
nanoassemblies is shown in Fig.
4.7
.
The determination of the number of dye molecules per QD over the course of
titration experiments is difficult, since the overall PL quenching depends both on the
(a priori unknown) quenching efficiency and on the number of dye molecules on the
QD surface. To separate these two effects, one needs the independent identification
of the number of attached H
2
P molecules merely from spectroscopic means. For the