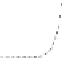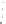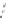Biomedical Engineering Reference
In-Depth Information
a
b
1
1,0
5,6
1
0,8
5,6
0,6
0,4
5,6
1
1
0,2
0,0
580
600
620
640
660
680
700
720
740
760
Wavelength, nm
Fig. 4.8
Soret band absorption and fluorescence spectra (normalized to the maximum) for
porphyrin (m-Pyr)
4
H
2
P molecules in absence and presence of CdSe (
a
)orCdSe/ZnS(
b
,
λ
420 nm) QDs in toluene at 295 K.
Inset
in
a
: position of the Soret band (with respect
to the Soret band of pure H
2
P) in presence of CdSe QDs at small molar ratios
x
.(
b)
1
(
x
=
exc
=
0.52,
λ
=
650 nm), 2 (
x
=
1.03,
λ
=
650 nm), 3 (
x
=
2.04,
λ
=
651 nm), 4 (
x
=
4.02,
max
max
max
λ
=
651 nm), 5 (
x
=
7.5,
λ
=
652 nm), 6 (pure porphyrin without QD,
λ
=
653 nm)
max
max
max
“QD-H
2
P” nanoassembly formation, the spectra show that only a small part of
H
2
P molecules added during the titration procedure becomes attached to the QD
surface, indicated by the missing of an isosbestic point [
64
]. It should be mentioned
that noticeable spectral shifts in the PL of the QDs were not observable upon
nanoassembly formation in any case.
On the other hand, for the H
2
P molecules being attached on QD surface,
spectral red shifts of both the Q- and Soret absorption bands and a blue shift of
the fluorescence Q-band accompanied by a slight change in the Franck-Condon
envelope of the overall spectrum are observed [
62
,
101
](Fig.
4.8
).
These shifts and the slight inhomogeneous broadening of the porphyrin Soret
band indicate that at low molar ratios
x
most of the H
2
P molecules are conjecturally
complexed in “QD-H
2
P” nanoassemblies. There are several possible explanations
for the observed spectral shifts. In principle, the spectral properties depend on
several factors such as the molecular geometry, QD surface composition, the
overall dielectric function, and polarization effects via the
meso
-pyridyl rings upon
attachment to the QD surface. A red shift (both for absorption and for emission)
is expected in relation to an increase of the average dielectric constant which is
approximately
2.4 for toluene. On the contrary, the Q-
band emission exhibits a blue shift which cannot be explained by a dielectric model.
The Stokes shift between Q-band absorption and emission becomes smaller upon
assembly formation. At the same time, the electronic Q(0,0)-band intensity of H
2
P
(
ε
=
9.7 for CdSe and
ε
=
653 nm) is reduced relative to Q(0,1) vibronic one (at 720 nm) which indicates
that the Franck-Condon factor is changing upon assembly formation. It is known
that the Q-bands in H
2
P correspond to symmetry-forbidden transitions [
122
], which
will become partly allowed by distortion of the H
2
P ring upon attachment to the
QD. These facts induce an increased Stokes shift and an increase of the formerly
λ
=