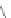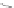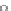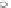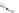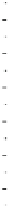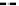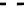Biomedical Engineering Reference
In-Depth Information
a
b
2
- (m-Pyr)
2
1
-
(
o-Pyr)
4
1,0
1,0
2
- (m-Pyr)
2
1
- (o-Pyr)
4
3
- (m-Pyr)
1
0,8
3
- (m-Pyr)
1
0,8
4
- (m^Pyr)
2
4
- (m^Pyr)
2
0,6
0,6
6
- iso(m-Pyr)
3
0,4
6
- iso(m-Pyr)
3
0,4
5
- (m-Pyr)
3
5
- (m-Pyr)
3
0,2
0,2
7
-
(
m-Pyr)
4
8
- (p-Pyr)
4
8
- (p-Pyr)
4
7
- (m-Pyr)
4
0,0
0,0
012345678910111213141516
0
2
4
6
8
10
12
14
16
Molar ratio x
Normalized molar ratio x
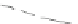
Fig. 4.6
Relative PL intensity changes (quenching)
I
(
x
)/
I
(0) as function of the molar ratio
x
[
C
Porph
]/[
C
QD
](
a
) and the normalized (to the number of pyridyl groups) molar ratio
x
(
b
)for
CdSe/ZnS QD (
d
CdSe
=
=
10
−
7
M) and various porphyrin molecules:
(
1
)(
o
-Pyr)
4
-H
2
P; (
2
)(
m
-Pyr)
2
(Ph)
2
-H
2
P; (
3
)(
m
-Pyr)
1
-H
2
P; (
4
)(
m
ˆPyr)
2
-H
2
P; (
5
)(
m
-Pyr)
3
-H
2
P;
(
6
) (iso)-(
m
-Pyr)
3
-H
2
P; (
7
)(
m
-Pyr)
4
-H
2
P; (
8
)(
p
-Pyr)
4
-H
2
P. Toluene, 295 K. Adapted from [
62
]
2.5 nm,
n
ZnS
=
2,
C
QD
=
4
×
substitutes when changing from (
m
-Pyr)
1
to (
m
-Pyr)
4
, with one exception, namely
(
m
-Pyr)
2
which shows an overall behavior more similar to (
m
-Pyr)
1
, whereas
(
m
ˆPyr)
2
shows a much more efficient quenching similar, e.g., to (
m
-Pyr)
3
.
AsshowninFig.
4.6
a, the QD PL quenching efficiency and thus the probability
to form QD-H
2
P nanoassemblies is decreased with a decreasing number of pyridyl
rings. Assuming that the probability of the nanoassembly formation is linearly
proportional to the number of pyridyl rings, one can define an effective molar
ratio
x
pyr
x
(
N
/4) that scales with
N
,where
N
is the number of pyridyl rings for
agivenH
2
P molecule. Correspondingly,
x
pyr
becomes smaller with a decreasing
number of pyridyl rings. Doing so, we obtain a rescaling of the QD PL quenching
efficiency for every H
2
P molecule [
62
] (depicted in Fig.
4.6
b). In the result, all
of the quenching curves besides those for (
m
-Pyr)
1
-H
2
P, (
m
-Pyr)
2
(Ph)
2
-H
2
Pand
(
o
-Pyr)
4
-H
2
P are shifted towards one single curve. The overall result is a kind of
“master” curve for the quenching efficiency. In case that only one pyridyl ring
can be anchored effectively, the agreement with the master curve becomes less
satisfactory. It follows from this behavior that the assumption relating the probability
to form a QD-H
2
P nanoassembly with the number of pyridyl rings having access
to the QD surfaces is correct. The stability of a two-point interaction will be at
least a factor of 2 stronger than a one-point interaction, as can be deduced from the
pronounced mismatch of the (scaled) one-point interaction curves for (
m
-Pyr)
1
-H
2
P,
(
m
-Pyr)
2
(Ph)
2
-H
2
P as compared to the master curve. The importance of a two-point
interaction has also been demonstrated for CdSe/ZnS QD-protein complexes [
121
].
The variation of the QD PL quenching efficiency with respect to the number, kind,
and position of pyridyl rings in H
2
P molecules points toward a dynamic equilibrium
between QD-H
2
P nanoassemblies and free entities, as has also been observed for
multiporphyrin arrays [
107
,
108
,
112
,
113
]. The equilibrium is dynamic, since
=