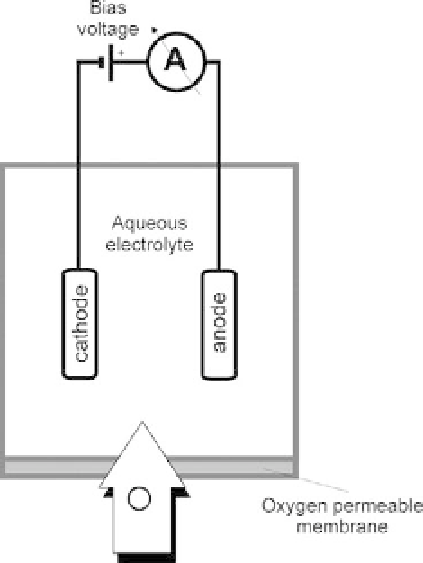
Biomedical Engineering Reference
In-Depth Information
O
2
sensor, also widely known as a Clark electrode, is used to measure the partial
pressure of O
2
gas in a sample of air or blood. This sensor is categorized as an amperomet-
ric (i.e., the measurement is based on the production of a current when a voltage is applied
between two electrodes) sensor and requires an external polarizing bias voltage source. The
measurement is based on the principle of polarography, as shown in Figure 10.27. The elec-
trode utilizes the ability of O
2
molecules to react chemically with H
2
O in the presence of
electrons to produce hydroxyl (OH
) ions. This electrochemical reaction, called an oxida-
tion/reduction or redox reaction, generates a small current and requires an externally
applied constant polarizing voltage source of about 0.6V.
Oxygen is reduced (consumed) at the surface of a noble metal (e.g., platinum or gold)
cathode (the electrode connected to the negative side of the voltage source) according to
the following chemical reaction:
A
p
4OH
In this reduction reaction, an O
2
molecule takes four electrons and reacts with two water
molecules, generating four hydroxyl ions. The resulting OH
ions migrate and react with
a reference Ag/AgCl anode (the electrode connected to the positive side of the voltage
source), causing a two-step oxidation reaction to occur as follows:
Ag
4e
$
O
2
þ
2H
2
O
þ
Ag
þ
þ
e
$
Ag
þ
þ
Cl
$
#
In this oxidation reaction, silver from the electrode is first oxidized to silver ions, and
electrons are liberated to the anode. These silver ions are immediately combined with
AgCl
FIGURE 10.27
Principle of a polarographic Clark-type
p
O
2
sensor.