Biomedical Engineering Reference
In-Depth Information
chloride ions to form silver chloride that precipitates on the surface of the anode. The cur-
rent flowing between the anode and the cathode in the external circuit produced by this
reaction is directly (i.e., linearly) proportional to the number of O
2
molecules constantly
reduced at the surface of the cathode. The electrodes in the polarographic cell are immersed
in an electrolyte solution of potassium chloride and surrounded by an O
2
-permeable Teflon
or polypropylene membrane that permits gases to diffuse slowly into the electrode. Thus,
by measuring the change in current between the cathode and the anode, the amount of oxy-
gen that is dissolved in the solution can be determined.
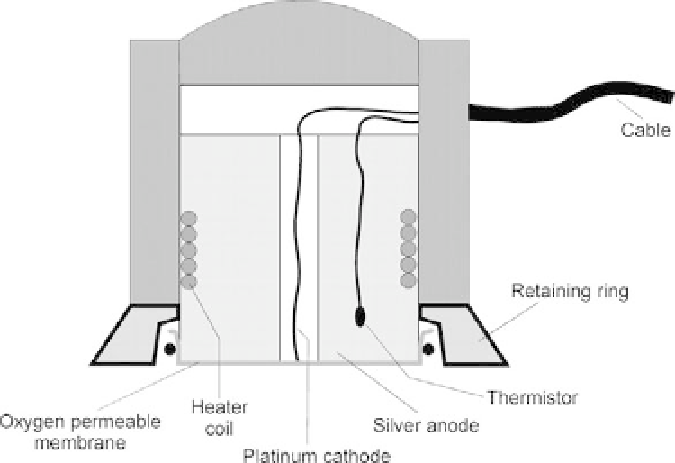
With a rather minor change in the configuration of a polarographic
p
O
2
sensor, it is also
possible to measure the
O
2
transcutaneously. Figure 10.28 illustrates a cross section of a
Clark-type transcutaneous
p
p
O
2
sensor. This sensor is essentially a standard polarographic
p
O
2
electrode that is attached to the surface of the skin by double-sided adhesive tape. It
measures the partial pressure of oxygen that diffuses from the blood through the skin into
the Clark electrode, similar to the way it measures the
O
2
in a sample of blood. However,
since the diffusion of O
2
through the skin is normally very low, a miniature heating coil is
incorporated into the housing of this electrode to cause gentle vasodilatation (increased
local blood flow) of the capillaries in the skin. By raising the local skin temperature to about
43
C, the
p
O
2
measured by the transcutaneous sensor approximates that of the underlying
arterial blood. This electrode has been used extensively in monitoring newborn babies in
the intensive care unit. However, as the skin becomes thicker and matures in adult patients,
the gas diffusion properties of the skin change significantly and cause large errors that
result in inconsistent readings.
Various methods for measuring the oxygen saturation, SO
2
(the relative amount of oxy-
gen carried by the hemoglobin in the erythrocytes), of blood in vitro or in vivo in arterial
blood (S
a
O
2
) or mixed venous blood (S
v
O
2
), have been developed. This method, referred
to as oximetry, is based on the light absorption properties of blood and, in particular, the
p
FIGURE 10.28
Transcutaneous
p
O
2
sensor.