Environmental Engineering Reference
In-Depth Information
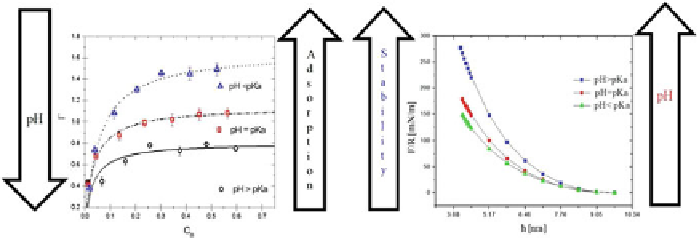
Fig. 10
Fully charged linear cationic poly-electrolyte on neutral surfaces with
N
=
7 beads. Only
every other bead is charged. The
left panel
shows the adsorption isotherms obtained at three different
pH values. The symbols represent the results of the simulations while the
solid lines
are the best
fits to the Langmuir adsorption isotherm model. In the
right panel
of this figure we show the full
force between colloidal surfaces mediated by the poly-electrolytes and the solvent, for the same pH
values as in the
left panel
. Notice that when the pH is increased so is the surface force, in contrast
with the adsorption isotherm trend. The axes in the
left
figure are shown in reduced DPD units while
those on the
right
have been appropriately dimensionalized. Adapted from Alarcón et al. (
2013a
)
electrolyte used in those simulations the adsorption increases as the pH is reduced,
which was attributed to the competition between the poly-electrolytes and their coun-
terions for the adsorption sites on the colloids surfaces, because the adsorption of
the counterions grows when that of the poly-electrolytes is reduced. This is precisely
the trend found in experiments performed on comparable situations (Drechsler et al.
2010
). The right panel in Fig.
10
shows the full surface force that neutral colloidal
particles, modeled by the exact DPD wall force, exert on each other by means of
the cationic poly-electrolytes at different pH values (Alarcón et al.
2013a
). This is
the first calculation of its kind, not only within the context of DPD simulations. The
trend found in the surface force is entirely different from that found in the adsorption
isotherms (left panel in the same figure) even though the calculations were performed
on the same systems. In other words, when the pH of the cationic poly-electrolyte is
increased, it translates into a larger surface force between colloidal particles. What
this means is that if one is looking for optimal stability of colloidal dispersions it
may be advantageous to add less poly-electrolytes as dispersants, at a basic pH,
because the competition of electrostatic interactions between poly-electrolytes and
counterions, and the excluded volume interactions with the solvent are enough to
increase the many-body surface force so that the colloidal dispersion turns out to be
optimally stable, as the right panel of Fig.
10
shows. The results shown in the right
panel of Fig.
10
fully reproduce the experiments carried out on poly-electrolytes and
characterized using atomic force microscopy at different pH (Drechsler et al.
2010
),
among others.
Lastly, we comment on some very recent DPD simulations that model complex
fluids under stationary flow using soft DPD potentials. The reason for simulations
of this type stems from the need to understand phenomena seen in applications such
as drug-carrying liposomes in the pharmacological industry, in the processes of
Search WWH ::

Custom Search