Environmental Engineering Reference
In-Depth Information
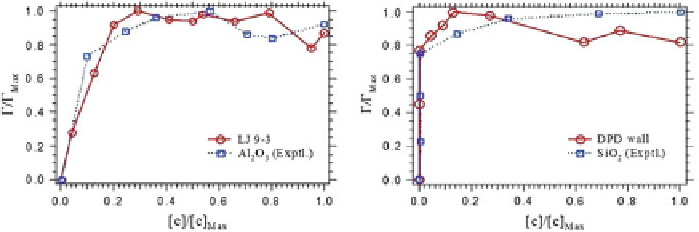
Fig. 9
Adsorption isotherms obtained with GCMC-DPD simulations of fluids confined by a
Lennard-Jones (9-3) surface model (
left
), and a linearly decaying, DPD wall force (
right
). The
fluid is made up of the monomeric solvent and a varying number of linear polymer chains (N
=
7)
to represent PEG molecules.
Red circles
represent the simulation results while the
blue squares
are the experimental data as taken from Esumi et al. (
2001
). The axes are normalized with their
maximum value so that both scales range from 0 to 1. The
lines
are only guides for the eye. Adapted
from Gama Goicochea (
2007
)
beads each, joined by harmonic springs. This polymerization degree corresponds to
a molecular weight
M
w
400 for
PEG
(Gama Goicochea
2007
). The predicted
adsorption isotherms are compared with the experimental counterparts (Esumi et al.
2001
)inFig.
9
. Notice how the DPD methodology correctly reproduces the trends if
not the actual values of the isotherms; not only that, it is possible to model different
surfaces characteristics by a judicious choice of the wall force model or interaction
parameters. Up to this point all the results reported for confined fluids were carried
out for neutral systems. However, poly-electrolytes which are charged polymers
are ubiquitous in nature and in modern day applications (Holmberg
2003
). Many
colloids acquire electric charges on their surface when immersed in a polar solvent,
like water, and are rich in showing complex phenomena when they are subject to
varying ionic strength and pH, as poly-electrolytes are as well. From the point of
view of fundamental research, the long range nature of the Coulomb interaction gives
rise to behavior that is qualitatively different from neutral systems, which needs to
be thoroughly investigated to reach a satisfactory understanding of soft condensed
matter systems. Because of these needs it became necessary to adapt the DPD model
so that it could handle long range interactions such as the electrostatic one, for
confined systems. The natural route was to adapt the Ewald sums method for cases
when there is reduced symmetry, as in the confined fluids we have discussed (Alarcón
et al.
2013a
).
Alarcón and co-workers (
2013a
) calculated the first adsorption isotherms of poly-
electrolytes using the GCMC-DPD algorithm adapted with Ewald sums for confined
systems as a function of pH. They studied the adsorption of weakly charged, linear
cationic and anionic poly-electrolytes at various values of pH on negatively charged
and neutral colloidal surfaces. The adsorption isotherms they obtained for cationic
poly-electrolytes adsorbed on neutral surfaces modeled by the exact, self-consistent
DPD wall force (see Eq.
14
) are shown in the left panel of Fig.
10
. For the model poly-
=
Search WWH ::

Custom Search