Biomedical Engineering Reference
In-Depth Information
(a)
(b)
Au
mmPA image
1. PL-PEG-COOH
Au NR
MNP-Au
MNP
HAuCl
4
MNP
MNP
MNP
2. PLH
NH
2
OH
(d)
(c)
PEG
(e)
Clustered
MNPs
Single
MNP
900 nm, 80 mJ cm
−2
Magnet
300
Polymer
coating
200
30 µm
MNP
MNPs
Iron
core
100
Cell
0
Human AT F
Magnet on 1 h
Magnet
removed
No magnet
figure 10.13
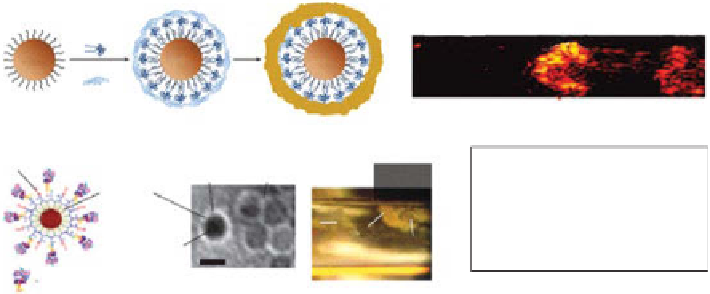
Magnetomotive photoacoustic (PA) contrast agents. (a) Schematic of
magnetic nanoparticle-gold core-shell nanostructures (MnP-Au) (reprinted with permission
from ref. [147]. © Macmillan Publishers Ltd.). (b) Magnetomotive PA imaging of gold
nanorods (Aunr), MnP-Au, and magnetic nanoparticles (MnPs). (c) Schematic (left) and
TEM image (right) of MnPs. (d)
In vitro
magnetic trapping of MnP-labeled cancer cells at a
flow velocity of 5 cm/s. (e)
In vivo
PA detection of magnetic enrichment of circulating tumor
cells in tumor-bearing mice. (reprinted with permission from ref. [148]. © Macmillan
Publishers Ltd.) (
See insert for color representation of the figure
.)
For the second method, MnPs were aggregated under a magnetic field, the local
concentration of MnPs within an roI was increased, and finally, the PA signals were
significantly enhanced, referred to as magnetic enrichment [147]. This approach was
applied to magnetically capture rare circulating tumor cells (CTCs) in the systemic
bloodstream in living mice and photoacoustically detect the CTCs with high contrast.
The illustration and TEM image of MnPs are shown in Figure 10.13c. Magnetic cap-
turing of CTCs conjugated with MnPs was clearly identified as shown in an optical
microscopy image
in vitro
(Fig. 10.13d). PA signals measured from CTCs in abdom-
inal skin vessels of tumor-bearing mice were compared before, during, and after
magnet placement. The PA amplitude increased 88-fold (Fig. 10.13e). After the
magnet was removed, the PA signals were dramatically reduced due to the release of
captured CTCs attached to MnPs. As another example, plasmonic gold nanostars
cored with superparamagnetic iron oxide were effectively used as a sentinel lymph
node tracer in PAT [114].
As another example, Kircher
et al
. have recently developed a novel triple-modality
MrI-PA imaging-raman imaging nanoparticle (MPr nanoparticle) to clearly visu-
alize the brain tumor margins [149]. The MPr nanoparticle comprised a 60 nm gold
core covered with the raman molecular tag, and a 30 nm silica coating protected the
raman-active layer. Further, the particles were coated with gd
3+
ions resulting in the
MPr nanoparticle (Fig. 10.14a). The clinical scenario includes (i) preoperative MrI
detection of brain tumors after injection of MPr nanoparticles and its consequent
surgical planning, (ii) intraoperative PA image-guided surgical resection of bulky
tumors, and (iii) raman image-guided microsurgery of residual tumors (Fig. 10.14a).









Search WWH ::

Custom Search