Biomedical Engineering Reference
In-Depth Information
(a)
(b)
Enzymatic cleavage
MMP
-2
Au
cage
Au
cage
C2
C1
C1
Protein cleavage site
Subtracted
675 nm
750 nm
(c)
0
0
0.5
1
1.5
2
2.5
3
3.5
4
4.5
5
0
4.5
0.06
Photoacoustic
signal
0.08
0.5
4
0.5
0.05
1
1.5
2
2.5
0.07
3.5
3
2.5
2
1.5
1
0.5
0
λ
C1
λ
C2
λ
C1
B-APP-A
0.06
0.04
0.4
0.05
0.03
0.3
0.04
0.03
0.02
0.01
0
3
3.5
4
4.5
5
0
0.02
0.2
B-CP
0.01
0.1
0
0
1
x(cm)
2
1
2
0
1
2
x(cm)
x(cm)
figure 10.12
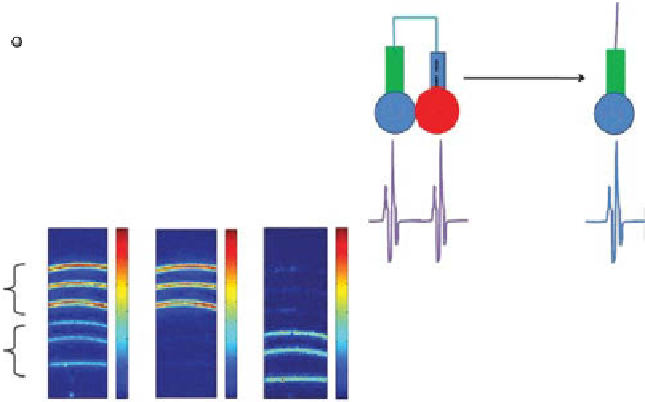
Enzyme-sensitive activatable photoacoustic (PA) contrast agents. (a) Schematic
of a gnC-based activatable probe. gnCs and fluorescent dyes are linked through enzyme-
cleavable peptides (based on ref. [146]). (b) Schematic of an activatable PA probe consisting of
two chromophores linked through enzyme-cleavable peptides. Initially, two strong PA signals
were generated at two optical wavelengths. When cleaved, the PA signal was induced at only
one of the two wavelengths. (c) dual-wavelength PA imaging of the activatable probes.
B-APP-A (BHQ3-APP-Alex 750, uncleaved) and B-CP (cleaved). APP, activatable photo-
acoustic probe. (Adapted with permission from ref. [145]. © American Chemical Society.)
10.5.6 multifunctional magnetomotive Photoacoustic
contrast agent
As another approach to boost PA sensitivity, multifunctional magnetomotive PA
(mmPA) contrast agents were successfully developed [147-149]. Strong background
PA signals from intrinsic contrasts can be significantly suppressed by either magnetic
modulation or magnetic enrichment. For the first method, magnetic nanoparticle-gold
core-shell nanostructures (MnP-Au) were manipulated as shown in Figure 10.13a
[148]. The principle of mmPA imaging was that MnP-Au nanoparticles were dis-
placed under a magnetic field and then they returned back to the original positions
when the field was off. By processing a series of PA images based on the magnetic-
induced motion, PA signals stemming from MnP-Au nanoparticles were rectified,
while all background signals were rejected. This process greatly improved the PA
sensitivity and specificity of the nanoparticles. Figure 10.13b shows that mmPA imaging
significantly improved contrast sensitivity and specificity. Although an inclusion con-
taining gold nanorods generated strong PA signals in a conventional PA imaging mode,
the PA signals were completely suppressed in the mmPA imaging mode. However, the
strong PA signals were produced from an inclusion with MnP-Au nanoparticles in
both conventional and mmPA imaging modes and also stronger (20 dB) than that with
MnPs alone. In addition, these MnPs can be used as an MrI contrast agent as well.





















































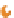

Search WWH ::

Custom Search