Biomedical Engineering Reference
In-Depth Information
100
90
Absorption
Emission
80
70
60
50
40
30
20
10
0
500
550
600 650
Wavelength (nm)
700
750
800
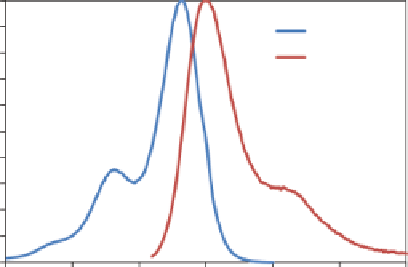
FIgure 9.2
excitation and emission spectrum of Alexa Fluor 633 fluorescent dye.
The characteristics of a fluorophore are usually defined by molar extinction coeffi-
cient (molar absorptivity), maximum excitation (absorption) wavelength, maximum
emission wavelength, quantum yield, and lifetime. The maximum excitation wavelength
determines the penetration depth of photons inside the tissue. The
molar extinction
coefficient
demonstrates the light absorption capability of a fluorophore. Fluorophores
with a higher molar extinction coefficient have stronger capability to absorb photons.
The
fluorescence quantum yield
of a fluorophore is the ratio of the number of emitted to
the number of absorbed photons. Fluorescent probes need to have high quantum yield
to generate a brighter fluorescence signal.
Fluorescence intensity
is proportional to the
product of its extinction coefficient and quantum yield.
Fluorescence lifetime
is the
average time that an excited fluorophore stays in the excitation state before its transition
to the ground state followed by the emission of a photon. excited state lifetimes can
change with fluorophore microenvironment.
Fluorescence quenching
refers to any events that can decrease the fluorescence
intensity of a fluorophore. It can be caused by a wide variety of processes and is
often categorized as dynamic and static quenching. Dynamic quenching happens
when a fluorophore is in contact with another molecule that acts as a quencher and
when quenching happens by transient collisional interaction between excited state
of fluorophore and ground state of quencher. In this case, the excited state of fluoro-
phore overlaps with the quencher's ground state, and its electron at the excited state
transfers its energy nonradiatively to the quencher's ground state and moves the
electron to quencher's excited state. In this case, the energy can transfer from the
quencher's excited state to its ground state as nonradiative (dark) or as a photon at
the quencher's emission wavelength. The final product acts as a fluorescence agent
with excitation emission spectra of an original fluorophore and emission spectra of
a quencher (Fig. 9.3). Dynamic quenching is extremely dependent on the molecular
distance between fluorophore and quencher. It typically occurs in distances up to
100Å. oxygen, halogens, amines, iodide ions, and electron-deficient molecules like
acrylamide are common chemical quenchers.
Search WWH ::

Custom Search