Biomedical Engineering Reference
In-Depth Information
Excited singlet state
S
2
S
1
Excited triplet state
Fluorescence
Phosphorescence
S
0
Ground state
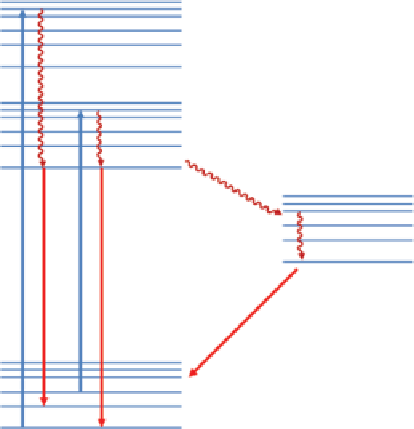
FIgure 9.1
Jablonski diagram.
shows the Jablonski diagram, which demonstrates the different transitions of an elec-
tron after absorption of a photon [28].
A photon can be absorbed by an electron in the ground state and transfer the elec-
tron to a higher energy state in the excitation band. This transition is very fast and
usually takes about 10
−15
s. If the electron in the excited state spins in the opposite
direction of the electron in the ground state, it can return back to the ground state
rapidly by emission of a photon. This phenomenon is called fluorescence, and its
emission time is between 10
−9
and 10
−7
s. If the excited electron is in triplet state and
has the same spin direction of the electron in the ground state, then its return to the
ground state is forbidden and usually takes about 10
−3
-1 s until it returns to ground
state and releases its energy as a photon. This phenomenon is called phosphorescence.
The energy of electron transitions between different vibrational levels in a single
energy state is released as thermal energy.
In most cases, emitted photons have longer wavelength and lower energy than the
absorbed photons. However, when the excitation light is very intense, it is possible for
one electron to absorb two photons. This two-photon absorption can lead to emission
of a photon with a shorter wavelength than the originally absorbed photons.
excitation wavelength is usually independent of the emission wavelength.
Fluorescence spectrum, when plotted as a function of wavelengths, is generally a
mirror image of its absorption (excitation) spectrum (Fig. 9.2). This is due to the sim-
ilar spacing in the energy structures of the ground and excited energy levels and the
intermediate vibrational relaxation of electron in the subbands of the ground and
excited states.
Search WWH ::

Custom Search