Biomedical Engineering Reference
In-Depth Information
sites decreases at each step of the synthesis, reducing the risk of incomplete
conversion or side reactions. Furthermore, purifications can be performed more
easily at every step (even though difficulty increases for high number of generation
dendrimers). The first examples of dendrimers produced by the convergent approach
were polyethers obtained from 3,5-dihydroxybenzyl alcohol grafted on a multifunc-
tional core [81]. since then, many other dendrimers have been produced by this
approach, as reviewed by Grayson and Fréchet [82].
In solution, the volume occupied by a dendrimer increases cubically with
generations, while the mass increases exponentially. This typical expansion confers
to dendrimer properties that differ from linear polymers, especially as the size
increases. For instance, while the intrinsic viscosity increases linearly with the size
of linear polymers, it reaches a maximum at a certain generation for dendrimers [83].
Furthermore, from the MRI point of view, the higher rigidity of these structures leads
to higher relaxivity as the number of generation increases, while the size of linear
polymers has a limited influence on relaxivity because of their high flexibility.
several studies show that the relaxivity per Gd
3+
increases with generations. For
instance, Bryant and coworkers measured the relaxivity of G5 to G10 pAMAM
dendrimers with Gd
3+
-dOTA conjugated to the free amino groups at the surface (i.e.,
from 118 kda with 96 Gd
3+
to 3000 kda with 1860 Gd
3+
). The ion relaxivity increased
from 30 mM
−1
s
−1
for G = 5 to 36 mM
−1
s
−1
for G = 7 (20 MHz, 23°C). However, a plateau
was reached beyond G = 7. It was observed that increasing the temperature in turn
increased relaxivity, indicating that the relaxivity was limited by a slow water exchange
[84]. The use of a complex with a higher water exchange, ethylenepropylenetriamine-
n,n,n′,n″,n″-pentaacetic acid (epTpA; see Chart 8.5), was proposed to circum-
vent this issue [85]. The results obtained with pAMAM dendrimers from G5 to G9
demonstrated a beneficial effect of this ligand, even if a decrease in relaxivity was
observed for G9. nonetheless, the fact that decreasing the temperature leads to an
increase in the relaxivity indicated that the water exchange rate was no longer the
limiting parameter. Furthermore, the same study highlighted the fact that the internal
motion of dendrimers also influences relaxivity, as demonstrated by the improved
relaxivity obtained at lower pH resulting from protonation of the amines and
subsequent higher rigidity of the structure. The latter results were confirmed by a study
published by Rudovsky
et al
. who demonstrated that the relaxivity of a negatively
OH
OH
RHN
O
O
HO
HO
O
O
-
O
O
-
O
-
O
O
O
NHR
HN
S
N
OH
PAMAM
HN
N
O
-
O
N
N
O
O
O
OH
-
O
Gd
3+
Gd
3+
O
-
O
-
O
N
NN
R =
O
O
O
O
-
O
-
O
RHN
O
O
n
O
OH
NHR
OH
EPTPA
Dendrimer with Gd-chelate at the barycenter
chaRt 8.5
dendrimers with improved characteristics discussed in the text.







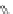
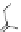

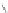
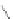

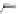


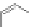
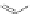











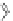
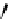
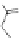




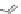
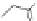




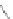
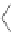






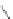
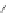








Search WWH ::

Custom Search