Biomedical Engineering Reference
In-Depth Information
(a)
(b)
C
O
OH
C
O
Cl
C
O
NHR
1
2345678
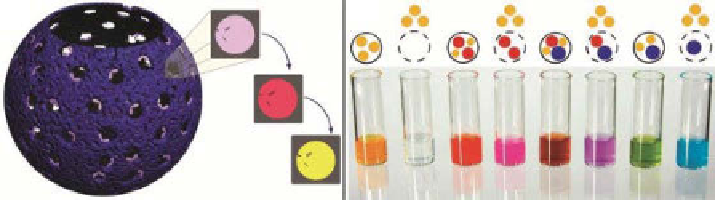
Figure 4.9
(a) The as-formed nanopores have a single carboxy functional group, which
can be converted into an acyl chloride group and then into an amide group. (b) Colored size
probe retention assay. NCs with encapsulated colored size probe mixtures were prepared and
separated on a size-exclusion column to remove released probes. Photograph of the NC frac-
tions: with encapsulated 0.6 nm (yellow) probes, (1) before template removal and (2) after
template removal, demonstrating complete release of the 0.6 nm probes; with encapsulated 0.6
and 1.1 nm (red) probes, (3) before template removal and (4) after template removal, demon-
strating complete release of the 0.6 nm probes and retention of the 1.1 nm probes; with encap-
sulated 0.6, 1.1, and 1.6 nm (blue) probes, (5) before template removal and (6) after template
removal, demonstrating release of the 0.6 nm probes and retention of the 1.1 and 1.6 nm
probes; and with encapsulated 0.6 and 1.6 nm probes, (7) before template removal and (8) after
template removal, demonstrating release of the 0.6 nm probes and retention of the 1.6 nm
probes. (Adapted with permission from Ref. [13]. © Wiley.)
To gain insights into the kinetics of transport through the shells of bilayer-
templated NCs, the rates of protonation and deprotonation of encapsulated pH-sensi-
tive dyes were investigated using stopped flow technique coupled with absorbance
and fluorescence spectroscopy [56]. In these experiments, an aqueous suspension of
surfactant-stabilized NCs containing pyranine or bromophenol blue was rapidly
mixed with a base or an acid followed by measuring fluorescence increase for the
deprotonated form of pyranine or absorbance decrease for the protonated form of
bromophenol blue. Remarkably, the rate of absorbance was the same for the encap-
sulated and free dyes (Fig. 4.10). These observations suggest that the transport
through the shell of the NCs is not the rate-limiting step in the overall process. Since
protonation/deprotonation reactions have very low energy barrier and are diffusion
controlled, we conclude that the mixing of two solutions is the rate-limiting step in
the overall process involving encapsulated pH-sensitive dyes. These observations
suggest that NCs can be used for real-time measurements of exterior ions or small
molecules. In addition, NCs with negligible mass transfer resistance may lead to fast-
acting devices for delivery of drugs or molecular imaging agents.
4.3 compartmeNtalizatioN oF molecules
iN Hollow NaNocapsules
molecules can be entrapped in hollow NCs during the synthesis stage. Alternatively,
larger molecules can be assembled from smaller components through a ship-in-a-
bottle assembly within prefabricated NCs (Fig. 4.11a) [57]. Another type of loading
Search WWH ::

Custom Search