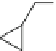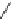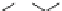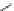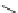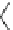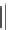Biomedical Engineering Reference
In-Depth Information
COOH
HOOC
N
COOH
N
HOOC
2
Tedisamil
4 Binding Mode Studies
Due to the absence of a determined 3D structure, researchers have to focus on the
binding modes investigation of different blockers to the transmembrane region of
K
v
1.5
potassium channel by mutagenesis studies and computer modeling.
Through site-directed mutagenesis studies, the preliminary mode of ligand-
K
v
1.5
binding was disclosed. Multichannel blockers such as Quinidine, Bupi-
vacaine and Benzocaine were located in a strong hydrophobic environment
consisting of Thr507, Leu510 and Val514 of the S6 domain that lines the inner
vestibule of the channel, and Thr479 near the selectivity filter, while Class I
a
antiarrhythmic agent Disopyramide interacted with Val512 residue in the Pro-
Val-Pro triplet amino acid region [
108
-
110
].
O
CH
3
NH
2
CH
3
NH
2
N
CH
3
N
N
N
H
3
C
O
H
3
C
CH
3
O
CH
3
O
CH
3
Bupivacaine
Benzocaine
Disopyramide
Recently, selective
K
v
1.5
blockers such as S-0100176 (37) and AVE0118 (24)
were employed in the mutagenesis studies as probes to explore the ligand-
K
v
1.5
interactions. It was concluded that residues including Thr479, Thr480, Val505,
Ile508, and Val512 that faced the central cavity were involved in the interaction
between 37 and
K
v
1.5
protein [
111
], whereas Thr479 and Thr480 in the selectivity
filter, as well as Ile502, Val505, Ile508, Leu510, Val512, and Val516 in the S6
domain formed a hydrophobic network for 24 [
112
]. Compared with 37, 24 was
prone to contacting with the open-state channel and stretching its conformation
to interact with critical hydrophobic Leu510, a residue predicted to face toward
S6 helix and away from the central cavity to strengthen its binding ability, named
“foot-in-the-door” phenomenon, while 37 was proposed to be trapped within the
central cavity in the close state (Fig.
5
).