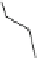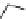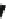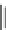Biomedical Engineering Reference
In-Depth Information
in converting AF to sinus rhythm (SR) in AF patients with a lower risk of pro-
arrhythmia [
103
].
OH
O
O
H
3
C
CH
3
CH
3
O
N
N
O
NH
N
AZD7009
3.4.3 Azimilide
Azimilide is a novel class III antiarrhythmic agent, which is now in the pre-
registration phase in FDA for arrhythmic disease. The prescription of Azimilide
is still controversial. As
I
Kr
,
I
Ks
dual blockers, the
I
Ks
blockade effect may result in
reduced TdP risk, however, the efficacy of Azimilide was inconsistent. The exten-
sive testing of Azimilide in AF patients did not show any risk of increased mortality
or morbidity, which might provide sense of comfort in prescribing it for high-risk
patients [
104
]. But according to Pritchett's report in a large randomized clinical
trials of post-infarct patients, Azimilide neither increased nor decreased mortality
risk [
105
]. Otherwise, all the finished pharmacological evaluations did not demon-
strate statistically significant efficacy in reducing the risk of arrhythmia recurrence
in patients with heart disease, who suffered from atrial fibrillation and then
converted to sinus rhythm [
106
].
CH
3
N
O
Cl
N
N
O
N
N
O
Azimilide
3.4.4 Tedisamil
Tedisamil is also a class III agent developed by Solvay Pharmaceuticals. It is now in
pre-registration phase of AF therapy. As multiple potassium channels including
I
Kr
,
I
Ks
,
I
Kur
,
I
to
, and
I
KATP
, the agent could effectively terminate atrial fibrillation and
flutter symptom, at 0.4 mg/kg and 0.6 mg/kg of intravenous tedisamil versus
placebo, 41% and 51% conversion versus 7% with placebo were observed. How-
ever, QT prolongation and ventricular tachycardia were observed at high dose
[
107
]. Accordingly, its utility for chronic treatment of AF might be limited by the
combination of QT prolongation and sinus slowing that could result in TdP.