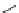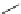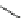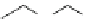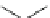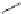Biomedical Engineering Reference
In-Depth Information
3.3.8 Triarylethanolamine Derivatives
Recently, Beshore et al. in Merck laboratory reported triarylethanolamine analogue
with enhanced positive ionizability to intervene
-cation interactions by intro-
ducing pyridinyl groups. Among these compounds, the most potent compound 41
(TAEA) reached an IC
50
of 0.28
p
M. However, further development was suspended
m
due to CNS side effects [
89
].
N
N
HO
N
TAEA
(41)
3.3.9 Pyrazolodihydropyrimidine Derivatives
In view of that, the
L
-type calcium antagonist Nifedipine was a weak blocker
of
K
v
1.5
current, Vaccaro and co-workers developed a series of dihydropyrazo-
lopyrimidine blockers [
90
]. The lead compound 42 showed moderate
K
v
1.5
blockade effect (IC
50
¼
1.1
m
M) and
L
-type calcium channel inhibitory effect
(IC
50
¼
M) with piperazine group instead
of ester group showed more potent inhibitory effect. Further modification showed
that dihydropyrazolopyrimidines with a C
6
heterocycle substituent possessed
high potency. The introduction of benzimidazole ring and the substituent in the
5-position of the dihydropyrazolopyrimidine ring produced 44 with an IC
50
of 0.03
6.1
m
M). Compound 43 (IC
50
¼
0.16
m
M without significant blockade of other cardiac ion channels [
91
].
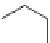
Then Lloyd et al. developed pyrrolidine amides of pyrazolodihydropyrimidine
compound BMS34136 (45), which was chosen for further in vitro and in vivo
evaluation [
92
].
m
Cl
Cl
Cl
O
H
Cl
O
N
N
N
N
O
N
CH
3
N
H
CH
3
H
CH
3
F
43
42