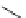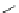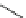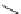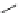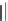Biomedical Engineering Reference
In-Depth Information
Cl
CH
3
N
OH
O
N
S
O
H
3
C
O
KN-93
(38)
3.3.7 Diisopropyl Amide Analogues
A novel type of blockers was discovered by Nanda et al. [
85
] through high-
throughput screening of the Merck sample collection [
85
-
88
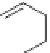
]. The lead compound
39 (IC
50
¼
M) with a main functional group of
N
,
N
-diisopropyl amide was
structurally distinct from recent disclosed cardiac channel antagonists. Based on the
successful case in the structural modification from AVE0118 (24) to S9947 (25),
the optimization strategy was focused on the two aryl rings. It was interesting
that the replacement of either of the phenyl rings with a 3-pyridyl ring was well
tolerated, but due to the different electrostatic interactions, incorporation of other
pyridine isomers resulted in a significant decrease in potency. Compounds con-
taining two pyridine rings, including those with retained single 3-pyridyl ring,
would lose potency when compared with the 3-pyridyl and phenyl analogues.
Substitute effects were also briefly investigated: the 3-bromophenyl or
4-cyanophenyl analogues exhibited better potency than the parent phenyls but the
2-cyano substitute group was not well tolerated.
The replacement of ester group in compound 39 by 2-fluorobenzyl urea leads
to compound 40 (IC
50
¼
0.25
m
M), which demonstrated the best potency
against
K
v
1.5
at IC
50
of 150 nM. This single active enantiomer was used for
all further evaluation. No changes at any doses in VRP or QT interval in animal
tests suggested a selective atrial effect of 40. Moreover, compound 40 is also a
P-glycoprotein (Pgp) substrate with low potential for central nervous system (CNS)
exposure.
0.15
m
H
3
C
H
3
C
H
3
C
CH
3
H
3
C
H
3
C
H
3
C
N
CH
3
H
N
N
O
F
O
N
H
NH
H
3
C
O
39
(IC
50
= 0.25 µM)
O
O
40
(IC
50
= 0.15 µM)