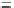Biomedical Engineering Reference
In-Depth Information
After the SAR analysis of different amide or sulfamide substitute groups, it was
found that the blockade effects would be influenced by the steric and electrostatic
effects of these substitutes [
83
]. The correlation between the inhibitory activities
and the p
K
a
was observed. The p
K
a value decreased from 7.5 of compound 34
to 6.2 of compound 35 (1.21-fold), accompanied by the IC
50
decreasing from 0.5 to
4
M (eightfold). Otherwise, replacement of the acidic hydrogen by a methyl group
(36, IC
50
¼
m
M) resulted in further reduction of activity. These results suggest
that the hydrogen might be involved in an intramolecular hydrogen bond with
the carbonyl oxygen, which stabilizes a favorable active conformation. The replace-
ment of methyl group of 36 to pyridine group lead to a potent
K
v
1.5
blockers
S-0100176 (37, IC
50
¼
10
m
0.7
M).
m
CH
3
CH
3
O
O
O
H
3
C
H
NH
S
H
NH
S
O
O
O
O
F
CH
3
F
F
34
35
CH
3
O
H
CH
3
N
S
O
O
36
N
O
N
HN
S
CH
3
O
O
S-0100176
(37)
Based on the above results, Rezazadeh et al. reported a new
K
v
1.5
blockers,
KN-93 (38,IC
50
¼
M), which showed multiion channel blockade effect,
including
K
v
1.2
,
K
v
1.4
,
K
v
2.1
,
K
v
3.2
, and
K
v
4.2
, and also an inhibitor of
Ca
2+
/calmodulin-dependent protein kinase II (CAMK-II) [
84
].
0.3
m