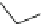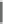Biomedical Engineering Reference
In-Depth Information
Cl
Cl
Cl
F
F
Cl
N
O
N
N
N
N
CH
3
N
N
N
H
H
CH
3
O
CH
3
44
BMS34136
(45)
3.3.10 Common Structural Features for K
v
1.5 Channel Blockers
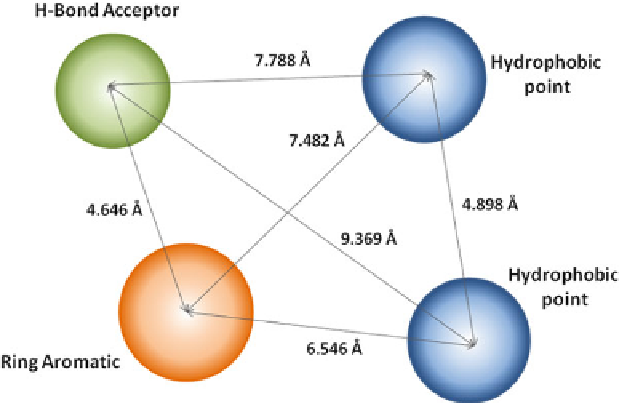
For the molecular simulation of protein-ligand interaction, a commonly used
method is to define three-dimensional arrangement of the structural and physico-
chemical features that are relevant to biological activity, or so-called pharma-
cophore identification. In the case of
K
v
1.5
blockers, prior reports had depicted a
three-hydrophobic-pharmacophore model [
71
,
81
]. However, the classic scaffold
was not matched with novel blockers and was not in accordance with the deve-
lopment of binding mode analysis. The up-to-date four-center pharmacophore
mapping for
K
v
1.5
blockers, including one aromatic ring, two hydrophobic points,
and a hydrogen-bond acceptor, was derived from forty compounds with distinct
structure types of
K
v
1.5
blockers (Fig.
4
). This recognition model, together with the
Fig. 4 Recently reported four point pharmacophore model for
K
v
1.5
blockers