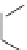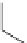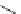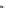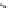Biomedical Engineering Reference
In-Depth Information
3.3.4 Quinoline or Isoquinoline Derivatives
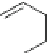
In 2005, Merck laboratories announced the patents of the quinoline and isoquinoline
derivatives (compounds 15 to 20)as
K
v
1.5
blockers [
59
-
67
]. ISQ-1 (15), obtained
by high-throughout screening approaches, was considered as lead compound [
68
].
The replacement of the dimethylaminomethyl moiety of ISQ-1 with a cyano group
could neutralize the alkalinity of amine and increase its selectivity over
h
ERG
potassium channel (21,IC
50
¼
M). Then the optimization was continued
to discover the ethanol amide compound 22, an improved potent
K
v
1.5
blocker
(IC
50
¼
0.07
m
M) with excellent selectivity over
h
ERG (over 500-folds) and good
pharmacokinetic properties (clearance 12 mL/min/kg; V
d
0.8 L/kg; oral bioavail-
ability 21%). When being evaluated in an in vivo canine electrophysiological model
in which
I
Kur
current is prominent in atrial repolarization [
69
], compound 22
(IC
50
¼
0.06
m
M) exhibited atrial refractory period (ARP) prolongation without
concomitant ventricular refractory period (VRP) prolongation. In consistent with
these evidences, injection of compound 22 to anesthetized dogs leads to selective
prolongation of AERP without side effect on VERP at any dose. Therefore, the
reasonable combination of pharmacokinetic properties and in vivo effects solidified
compound 22 as a promising atrial-selective agent for further investigation.
0.06
m
O
O
CH
3
N
N
N
CH
3
CH
3
H
3
C
H
3
C
O
O
O
F
ISQ-1
(15)
16
O
O
H
3
C
CH
3
N
O
H
3
C
O
N
F
17
O
N
H
3
C
O
N