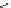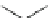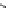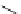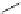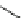Biomedical Engineering Reference
In-Depth Information
O
O
CH
3
N
O
H
3
C
N
CH
2
O
H
3
C
CH
3
O
N
O
F
19
20
OH
O
NH
O
CH
3
N
N
H
3
C
H
3
C
O
O
N
N
21
22
1, 1
0
-Disubstituted Biphenyl Compounds
3.3.5
Earlier in this century, Aventis started the rational design studies of selective
K
v
1.5
blockers. After analyzing the structural feature of reported
K
v
1.5
blockers
including the above-mentioned aryl sulfonamido indanes and tetrahydronaphthyls,
benzopyrans, thiazolidines, and quinolines, they concluded that the key structure
features for effective
K
v
1.5
blocker were two to three appropriate hydrophobic
points and flexible linkers between them. Then Peukert and collaborators launched
the 2D similarity search using aryl sulfonamido indanes as template to obtain a
1,8-disubstituted naphthalene scaffold (23,IC
50
¼
M). After chemical modi-
fication by replacing the framework with biphenyls, potent compounds AVE0118
(24,IC
50
¼
4.8
m
M),
and other analogues were obtained for further investigation. The SAR analysis of
this kind of
K
v
1.5
blockers showed some interesting results: first, the introduction of
pyridyl group in the side chains obtained higher activity than that of the phenyl ring,
aliphatic amine or hydrophobic chains (27-29,IC
50
¼
1.1
M), S9947 (25,IC
50
¼
0.4
M), S20951 (26,IC
50
¼
1.2
m
m
m
M,
respectively); on the contrary, the substitution of amide group with sulfamide group
that generally existed in the antiarrhythmic agents had slight influence on the
inhibitory effect (30,IC
50
¼
1.2
m
M, 2.2
m
M, 3.3
m
2.6
M) [
70
,
71
].
m