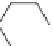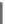Biomedical Engineering Reference
In-Depth Information
K
v
1.5
blocking and good selectivity over
h
ERG. The further modifications of the
benzopyran derivatives are still ongoing [
53
].
CH
3
CH
3
O
S
O
HN
H
3
C
HN
O
N
CH
3
CH
3
O
12
NIP-142 (13), a novel benzopyran derivative developed by Nissan, was able to
moderately block
hK
v
1.5
current (IC
50
¼
M) in a frequency-independent and
dose-dependent manner [
54
]. Electrophysiological evaluation showed that NIP-142
decreased phase I notch and increased the height of phase II plateau without making
any changes in APD [
55
]. Furthermore, NIP-142 could prolong AERP and APD
through blockade of
I
KACh
[
56
]. Briefly, NIP-142 has distinct pharmacological
properties from other classical antiarrhythmic agents, the overall biological features
may possibly contribute to its antiarrhythmic profiles as a promising agent for the
treatment of supraventricular arrhythmia [
57
].
4.75
m
O
H
3
C
HN
N
OH
CH
3
CH
3
O
O
O
2
N
NIP-142
(13)
Pyrano-[2,3
b
]-Pyridines
Based on the above results, Finlay reported a series of pyrano-[2,3
b
]-pyridine
compounds. However, the SARs of these compounds are differed from benzopyran
series, and the activities of pyrano-[2,3
b
]-pyridines are generally lower than
benzopyran compounds due to the introduction of polar groups. Among these
compounds, only 14 (IC
50
¼
0.39
M) showed moderate inhibitory effect against
m
K
v
1.5
channel [
58
].
CH
3
O
S
O
HN
O
OH
N
CH
3
CH
3
H
3
C
N
O
14