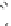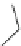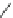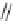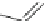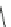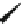Biomedical Engineering Reference
In-Depth Information
the cyclopropyl ring at the linker of the “lower” aromatic ring displayed potent
in vitro blockade potency against
K
v
1.5
(IC
50
¼
M) as well as atrial-selective
prolongation of AERP in vivo. Besides, it lacked hemodynamic side effects.
0.33
m
O
H
3
C
N
N
N
N
CH
3
4
2-Amino-2-Imidazolidinone Derivatives
Recently, Blass et al. reported a series of 2-amino-2-imidazolidinone compounds
to prevent the relative disposition of the aryl rings [
42
], the patents were also
announced [
43
-
45
]. Among this series of compounds, KVI-020 (5) showed suitable
physiochemical properties and was well tolerated in liver microsomal stability,
caco-2 permeability and protein binding tests. Further in vivo evaluation showed
that its pharmacokinetic and pharmaceutical properties were acceptable for late-
stage preclinical development.
O
H
3
C
O
N
N
NH
S
CH
3
O
O
H
3
C
O
5
In brief, the core heterocyclic ring thiazolidinone of ICA-32 could be extended
by its bioisosters including triazol, tetrazole, and imidazolidinone. However, the
inadequate additions of hetero atom in the rings or linkers lead to the undesired
metabolic and pharmacokinetic features, which hindered the future development of
ICA-32 analogues. Till now only 2-amino-2-imidazolidinones is in development.
3.3.2 Tetrahydroindolone Derivatives
Considering the unacceptable pharmacokinetic profile of ICA-32 analogues,
Wu and collaborators in P&G research laboratories developed another type