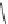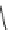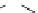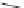Biomedical Engineering Reference
In-Depth Information
heterocyclic ring in the linker to form acylthiazolidines. Compound 2 (IC
50
¼
M)
was obtained through replacing “-CH
2
-” with “-O-” in the linker between core
heterocyclic ring and the upper ring with the rationale of “me-too” strategy.
However, these thiazoline compounds were rapidly metabolized. No parent com-
pound remained after 30 min incubation with rat S9 microsomal fraction because of
the oxidation of the sulfur atom in the heterocyclic ring [
39
].
0.09
m
O
H
3
C
O
O
N
S
easy to oxidized
H
3
C
CH
3
2
Triazol Derivatives
Based on the above results, 2,4-disubstituted-1,2,3-triazoles were synthesized to
avoid the rapid metabolism induced by sulfur atom oxidation [
40
]. Among these
compounds, compound 3 (IC
50
¼
M) with ketone linker between heterocylic
ring and lower ring was selected for further investigation. In the whole cell patch
clamp experimentation, significantly decreased potency was observed at
h
ERG
channel,
K
v
1.3
channel, and the L-type calcium channel. Moreover, 15 min infusion
at a dose of 30 mg/kg in an anesthetized pig model resulted in an increase of 12% in
the AERP, while the VERP remained unchanged.
0.29
m
O
H
3
C
N
N
N
O
H
3
C
CH
3
3
Tetrazole Derivatives
Except for triazol compounds, Wu described a replacement of the thiazolidinone
scaffold of ICA-32 with a tetrazole framework [
41
]. Compound 4 containing