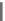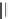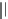Biomedical Engineering Reference
In-Depth Information
of
K
v
1.5
blockers based on tetrahydroindolone framework. However, only a
few compounds showed inhibitory effects against
K
v
1.5
. The in vivo assay of
tetrahydroindolone-derived semicarbazones 6 (IC
50
¼
M) showed an
increase in right AERP by 18% at a dose of 30 mg/kg without increase in VERP
[
46
]. To broaden the diversity of tetrahydroindolone-derived compounds, Fluxe
et al. prepared isosteric tetrahydroindolone-derived carbamates. The most potent
analogues, compound 7 (IC
50
¼
0.13
m
M) showed over 450-fold selectivity against
L-type calcium channel and
h
ERG channel (IC
50
>
0.07
m
M), in the meanwhile the
interestingly low activities against calcium and
h
ERG channels were also consistent
throughout the class [
47
].
30
m
CH
3
N
N
N
O
H
3
C
H
3
C
N
H
6
N
O
N
O
H
3
C
H
3
C
H
7
3.3.3 Aryl Sulfonamido Indane Derivatives
Aryl Sulfonamido Indanes and Tetrahydronaphthyls
Icagen and BMS reported a collection of aryl sulfonamido indanes and tetra-
hydronaphthyls with different amido region-isomers to determine the
K
v
1.5
pharmacophore [
48
-
52
]. It was reported that the (
1R
,
2R
) conformation of com-
pound 8 has a selectively high IC
50
of 0.03
M against
K
v
1.5
with high selectivity
m
over
h
ERG (inhibitory ratio: 37% at 10
M), while the
K
v
1.5
inhibitory IC
50
value
m
of (
1S
,
2S
) conformation was only 0.76
M. Aryl sulfonamido tetrahydronaphthyl
m
compound 9 (IC
50
¼
M) also showed good inhibitory effect against
K
v
1.5
channel. The modification of tetrahydronaphthyl scaffold lead to compound 10
(IC
50
¼
0.44
m
M), which underlies the replaceable of
rigid ring scaffold. However, the clinical trials of these compounds were suspended
due to the poor aqueous solubility and low oral bioavailability. In this series, the
chiral sulfonamide side chain was introduced, suggesting an asymmetry feature of
0.20
M) and 11 (IC
50
¼
0.24
m
m