Environmental Engineering Reference
In-Depth Information
Precombustion (decarbonization) capture
CO
2
Water
vapor
(and
excess air)
Mechanical
energy
CO
2
desorber
Hydrogen
Sulfur
removal
Steam
Gasifier
Particle
remover
Cooling
water
Steam
condenser
Heat
Nitrogen
Fuel
Oxygen
CO
2
absorber
Shift
reactor
Gypsum
Heat
recovery
steam
generator
Gas turbine
Fly ash
Steam
Mechanical
energy
Electricity
Bottom ash
Air
Air
Air
separation
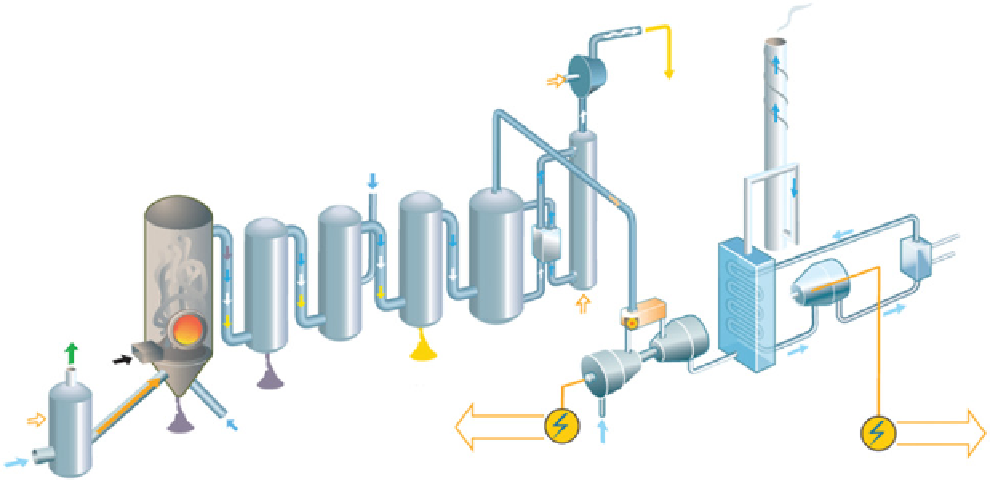
fiGure 21.8
precombustion schematic diagram flow. Courtesy from Ref. [17].
21.2.3
processes for Carbon Dioxide Capture
21.2.3.1 Precombustion Capture
Figure 21.8 shows the diagram of CO
2
removal using the precombustion process. The
system involves capturing CO
2
before burning the fuel. The fuel gas, liquid, or solid is first transformed into synthesis gas,
mainly CO and H
2
O, by reforming processes (for liquid and gaseous fuels) or gasification reactors (solid fuel). The latter
reaction is commonly called “partial oxidation” and proceeds according to reaction 21.1. The synthesis gas is cleaned to remove
particles that could damage the turbine or cause problems in subsequent steps of the process.
xy
+
CH HO CO
xy
x
+
x
+
H
(21.1)
2
2
2
2
Then CO is converted to CO
2
and H
2
by reacting CO with water vapor through a shift reaction (water-gas shift reaction):
CO HCOH
+
Ο
�
+
(21.2)
2
2
2
At this stage, the gas is composed mainly of CO
2
and H
2
. The concentration of CO
2
is about 15-60% (dry basis) and the total
pressure is typically between 2 and 7 mpa. CO
2
is removed by an absorption process. The typical solvents used for precombustion
capture are either physical (i.e., Selexol [18] and Rectisol) or chemical (i.e., methyldiethanolamine [19]). Unlike the solvents
for postcombustion capture, these technologies are mature and proven on a large commercial scale [20].
Although the initial fuel conversion steps of precombustion are costlier and more elaborate, the higher concentrations and
higher pressure of CO
2
in the gas stream make the separation and the subsequent compression of CO
2
easier than in postcom-
bustion. The removal rate of CO
2
is over 90%. In precombustion technology, the energy required for water-gas shift reaction,
solvent regeneration, and compression of CO
2
could represent anywhere from 14 to 25% of the output in a natural gas combined
cycle (NGCC) [21].
21.2.3.2 Postcombustion Capture
In this method, CO
2
is captured in a scrubber from flue gases that are close to atmospheric
pressure using an absorption process based on chemical solvents, such as amines (e.g., monoethanolamine). This is because
chemical solvents are less dependent on partial pressure than physical solvents, and the partial pressure of CO
2
in the flue gas
is low, typically 4-14% by volume. The gases pass through the absorption column where the solvent reacts with the CO
2
, chemically