Environmental Engineering Reference
In-Depth Information
Postcombustion capture (absorption process)
CO
2
Mechanical
energy
Low-
temperature
heat
Clean
flue gas
Steam turbine
CO
2
compressor
Electricity
Boiler
Cooling water
CO
2
stripper
Steam
condenser
Sulfur
removal
Particle
removal
Heat
CO
2
-lean
absorbent
Cooler
CO
2
-rich
absorbent
CO
2
absorbent
Air
fuel
Low-
temperature
heat
Fly ash
Gypsum
Bottom ash
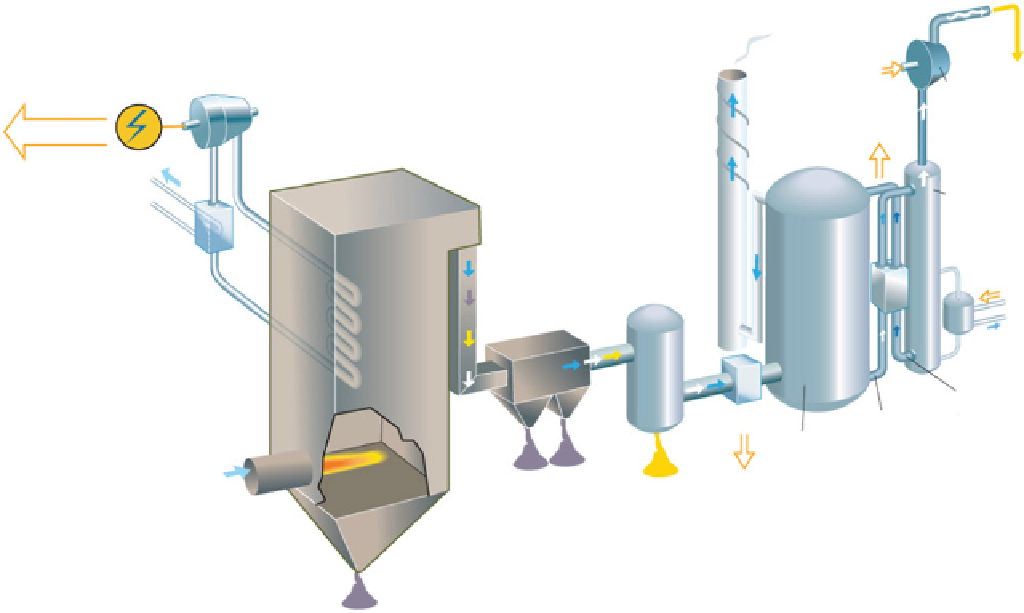
fiGure 21.9
postcombustion schematic diagram flow. Courtesy from Ref. [22].
binding it and removing it from the gas stream. After absorption, the cleaned flue gases primarily contain water vapor and
climatic inactive nitrogen (see Fig. 21.9). On leaving the scrubber, the solvent is heated to release the nearly pure CO
2
and the
solvent can then be reused (Fig. 21.10). Heat from the power plant's steam turbines is used to raise the temperature to the
desired level. The captured CO
2
can be transported to a storage site.
In the case of a power plant using postcombustion capture, the energy required for solvent regeneration and CO
2
compres-
sion could represent anywhere from 25 to 35% of its output.
21.2.3.3 Oxyfuel Combustion Capture
Oxyfuel combustion is still in the demonstration phase and uses high-purity
oxygen. This results in relatively higher CO
2
concentrations in the gas stream and, hence, in easier separation of CO
2
but
at the cost of increased energy requirements for the separation of oxygen from air. A large amount of oxygen is required
for combustion, which is obtained from an air separation unit. The flue gas from oxycombustion is compressed and
chilled to separate out nitrogen, oxygen, and other impurities. The resulting CO
2
concentration is typically 95 mol% or
more (Fig. 21.11).
21.3
Novel teChNoloGies
A major challenge in the implementation of carbon capture and storage (CCS) is that current industrial carbon capture
technologies are energy-intensive and not cost-effective [24]. To be viable, a capture technology must achieve 90% CO
2
capture with a maximum energy penalty of 10% [25]. The conventional technologies have many disadvantages: the
liquid-gas interaction, that is, the aqueous amine solutions, requires huge capital and operational costs because of the
energy needed for regeneration. Recently, gas-solid adsorption processes have been proposed as promising technologies
for carbon capture [26]. Solid sorbents have the potential to reduce the energy demand of capture processes because of
their potentially higher loading capacities, the absence of solvent heating and vaporization during regeneration, lower
material heat capacities, and lower heat of sorption [27-29]. These materials require a large surface area-to-mass ratio