Environmental Engineering Reference
In-Depth Information
CO
2
/CaCO
3
reactor
Flue gas
Captured and
compressed CO
2
Dispersal of
CO
2
/CaCO
3
mixture
Dispersal of CO
2
by ship
Refilling ship
Rising CO
2
plume
3
k
m
Sinking CO
2
plume
CO
2
lake
CO
2
lake
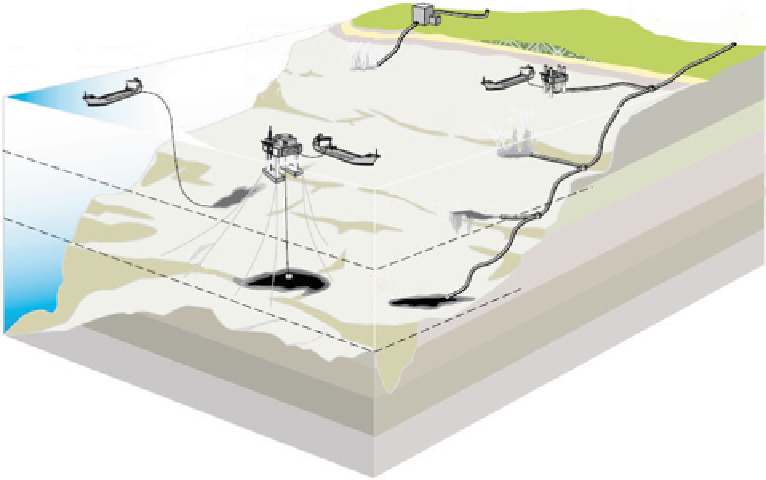
fiGure 21.7
Some of the ocean storage strategies for CO
2
. Courtesy from Ref. [13].
21.2.2
ocean storage
Captured CO
2
could be deliberately injected into the ocean at great depth, where most of it would remain isolated from the
atmosphere for centuries. CO
2
can be transported via pipeline or ship for release into the ocean or onto the sea floor.
The concept involves the slow exchange of CO
2
between the bottom water and the sea surface, allowing gas retention for
millennia.
Analyses of ocean observations and models agree that injected CO
2
will remain isolated from the atmosphere for several
hundred years and that the fraction retained will tend to be larger with deeper injection. Additional concepts to prolong CO
2
retention include forming solid CO
2
hydrates and liquid CO
2
lakes on the sea floor, as well as increasing CO
2
solubility by, for
example, dissolving mineral carbonates (Fig. 21.7).
21.2.2.1 Impacts on the Marine Environment
Overall, there is limited knowledge of the population in deep water, the
community structure, and the ecological interactions in deep water. The sensitivity of deep ocean ecosystems to store CO
2
remains largely unknown. most ocean storage proposals try to minimize the volume of water with high concentrations of CO
2
,
either by diluting the CO
2
in large volumes of water or by isolating the CO
2
in a small volume (e.g., lakes).
Ocean storage of CO
2
could occur deep in the ocean where there is virtually no light and a lack of photosynthetic organisms;
hence, those that could be affected are heterotrophic the organisms, that is, most animals. Therefore, the effects of CO
2
must be
identified at individual levels (physiological) of the ecosystem.
21.2.2.2 Mineral Carbonation
mineral carbonation results from the reaction of CO
2
with metal oxide-bearing materials,
the most attractive metals being calcium and magnesium. In nature, such a reaction is called silicate weathering and it takes
place on a geological timescale. However, in an industrial scenario, high concentrations of captured CO
2
are put into contact
with metal oxide-bearing materials, thus forming insoluble carbonates [15, 16].
The kinetics of natural mineral carbonation is slow; hence, all currently implemented processes require energy-intensive
preparation of solid reactants to achieve affordable conversion rates and/or additives that must be regenerated and recycled
using external energy sources. The resulting carbonated solids must be stored at an environmentally suitable location. The
technology is still in the development stage and is not yet ready for implementation. The best case studied so far is the wet
carbonation of the natural silicate olivine, which costs between 50 and 100 US$/tCO
2
stored and translates into a 30-50%
energy penalty on the original power plant.