Environmental Engineering Reference
In-Depth Information
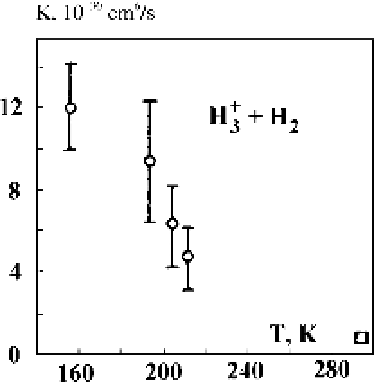
Figure 4.18
TherateconstantsforthethreebodyprocessH
3
$
H
5
according to
C
H
2
experimental data: open square - [77, 78], open circles - [79].
dencies for the recombination coefficient are due to the presence of vibrationally
excited ions in the beam. Next, the cross section
σ
rec
of dissociative recombina-
for H
3
10
13
cm
2
tion at electron energy
ε
D
0.01 eV is (1.1
˙
0.1)
ions and
10
13
cm
2
for D
3
ions according to measurements [83, 84]. Note that
processes of dissociative recombination involving molecular ions are of importance
in a stationary plasma and may establish therein the number density of electrons
and ions.
We briefly consider conversion between different types of ions in nitrogen. If
in the first stage of nitrogen ionization N
2
ions are formed, because of a high
dissociation energy of the nitrogen molecule only N
4
ions may be formed from
the initial ion in nitrogen at room temperature. Nevertheless, along with these ions,
N
C
and N
3
ions are also observed in nitrogen ionized by an external source. As
an example, Figure 4.19 gives the pressure dependence for nitrogen ions formed
in nitrogen if ions are created by an electron beam [85]. Note that transition from
nitrogen ions with an even number of nuclei to ions with an odd number of nuclei
is based on the process [86]
(0.5
˙
0.1)
N
2
(
4
N
3
C
Σ
u
)
C
N
2
!
N,
which includes electron excited state N
2
(
4
Σ
u
) of the nitrogen molecule. Hence,
nitrogen ions with an odd number of nuclei are formed in a source where ion-
ization proceeds by particles (e.g., by electrons or photons) whose energy exceeds
remarkably the ionization potential of the nitrogen molecule, as takes place un-
der the conditions in Figure 4.19. Next, an increase of the nitrogen pressure must
lead to an increase of the concentration of more complex ions, which follows from
Figure 4.19.
Note also that transitions between nitrogen ion pairs proceed weakly compared
with transitions between ion types inside one pair, so the total concentration of ions