Environmental Engineering Reference
In-Depth Information
room temperature. At higher gas temperatures or electric field strengths protons
may be present in hydrogen along with H
3
[69], whereas at low temperatures
ions with an odd number of nuclei, that is, H
5
and H
7
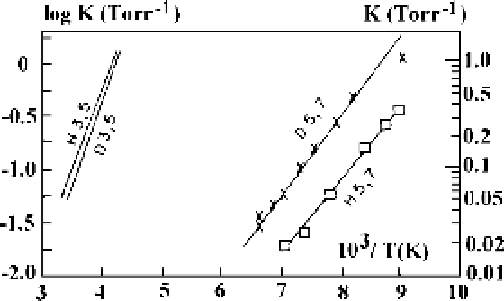
,maybepresent.Fig-
ure 4.17 shows the equilibrium constants for the processes H
3
C
H
5
,
H
2
$
D
3
C
D
7
obtained on the basis
of experiments [74-76]. In particular, from [76] it follows that under conditions of
thermodynamic equilibrium at a pressure of 1 Torr the number densities of H
3
and H
5
ions in hydrogen are equal at a temperature of 240 K, for D
3
and D
5
ions in deuterium the temperature at which the number densities of these ions are
equal is 236 K, for H
5
and H
7
ions in hydrogen this temperature is 115 K, and for
D
5
and D
7
ions in deuterium the temperature is 104 K. At a pressure of 10 Torr
the temperatures are 287, 279, 136, and 122 K, respectively.
Establishment of thermodynamic equilibrium between ions of different types re-
sults from conversion of simple ions into complex ones in three body collisions. In
particular, Figure 4.18 shows the rate constants for the three body process for for-
mation of H
5
from H
3
according to experiments [77-79]. As is seen, the rate con-
stant increases dramatically with decreasing temperature, and at room temperature
it is according to [77] 4.5
D
5
,H
5
C
H
7
,andD
5
C
D
2
$
H
2
$
D
2
$
10
31
cm
6
/s for formation of H
5
10
31
cm
6
/s
for formation of D
5
. One can see that in spite of the possibility of formation of var-
ious types of ions, a restricted number of ion types exists under certain conditions.
Processes of dissociative recombination of electrons and molecular ions may
be of importance for the existence of ions of a certain type in real ionized gas-
es. In the hydrogen case, the recombination coefficient of electrons and H
3
and 6.5
ions
10
7
cm
3
/s at room temperature [80], and for H
5
is 2.3
ions at 205 K the recom-
10
6
cm
3
/s [80]. The recombination coefficient mea-
sured for H
3
and D
3
ions in the temperature range 95-300 K is independent of
the temperature [81], whereas beam measurements give a remarkable dependence
of the cross section
bination coefficient is 3.6
σ
rec
of dissociative recombination on the electron energy
ε
,
1.3, for H
3
and D
3
ions in the energy range
0.01-1 eV [82]. It is quite possible that the different temperature and energy depen-
which is
σ
ε
n
,with
n
D
1.0
rec
Figure 4.17
The equilibrium constants for processes H
3
C
H
2
$
H
5
(H3,5), D
3
C
D
2
$
D
5
(D3,5), H
5
C
H
2
$
H
7
(H5,7), and D
5
C
D
2
$
D
7
(D5,7) [76].