Environmental Engineering Reference
In-Depth Information
O
+EtOH
-H
2
O
O
Ethylvalerate (EV)
O
O
+H
2
O
O
OH
γ
-Valerolactone (gVL)
Valeric acid (VA)
O
+H
2
-H
2
O
O
+2H
2
-H
2
O
OH
Pentylvalerate (PV)
OH
Pentanol
-H
2
O+H
2
O
2-Methyl-tetrahydrofuran (MeTHF)
FIGURE 18.11
Conversion of gVL to the VA biofuels EV and PV.
Valeric acid derivatives, such as ethyl valerate, are reported to have favorable
fuel properties (Lange et al., 2010). Noteworthy is the direct formation of pentyl
valerate (PV) from gVL over a bifunctional acidic and hydrogenation catalyst
(e.g., Pt/ZSM-5), as depicted in Figure 18.11. VA biofuels have acceptable energy
densities and a more appropriate (lower) polarity than, e.g., ethanol and butanol.
Depending on the chain length, they can be either employed for gasoline applications
(ethyl valerate (EV) and propyl valerate have boiling points around 150
C) or diesel-
range applications (PV, boiling point above 200
C). PV has a better volatility and
cold-flow property match with diesel than FAME, albeit with a lower energy density.
EV was evaluated positively in a road test, using a blend of 15 vol.% EV in regular
gasoline.
Example 18.4 Production of gVL
In the scheme depicted in Figure 18.12, a possible process integration concept for
gVL production is given (see Alonso et al., 2011).
Describe the advantages and disadvantages of this concept.
Answer
In this concept, the formic acid formed during cellulose hydrolysis to form LA is
used in the third step for the hydrogenation of LA to gVL. In this way, less addi-
tional hydrogen is needed. Secondly, streams of sulfuric acid are recycled, thus
making this, hopefully, a salt-free process.


























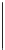














Search WWH ::

Custom Search