Environmental Engineering Reference
In-Depth Information
OH
OH
O
O
HO
O
O
HO
O
OH
n
Hydrogen
Cellulose
OH
Aqueous mixture:
levulinic acid +
formic acid + H
2
SO
4
Levulinic acid
+ solvent
Cellulose
hydrolysis
Hydrogenation
unit
Extraction
H
2
SO
4
/H
2
O
γ
-Valerolactone
+ solvent
Solvent recycling
Distillation
-Valerolactone
γ
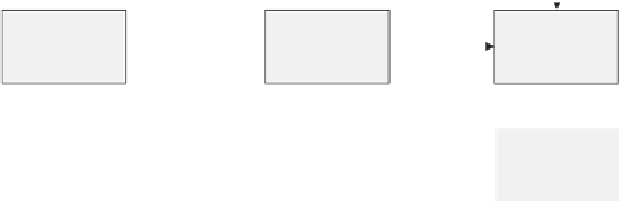
FIGURE 18.12
Process integration concept for gVL production.
Disadvantages of this process are the need for a solvent; solvent recycling
always leads to solvent losses, which are generally in the range of 20%. The hand-
ling of sulfuric acid streams is accompanied by environmental, corrosion, and
safety issues.
18.5 DIRECT AQUEOUS REFORMING OF SUGARS
LEADING TO A RANGE OF ALKANES
Aqueous-phase reforming (APR) of sugars and polyols was initially developed as a
technology to produce renewable hydrogen (+CO
x
). However, nowadays, by an
appropriate choice of catalyst, substantial amounts of light alkanes and monofunc-
tional compounds can be produced as well (Alonso et al., 2010).
To illustrate the principle of this technology, which is promoted by the company
Virent (tinyurl.com/q89rwxe), the equation is given for the conversion of glucose into
its fully saturated analogue hexane:
19C
6
H
14
O
6
!
13C
6
H
14
+36CO
2
+42H
2
O
ð
RX
:
18
:
1
Þ
The fact that no external hydrogen is needed makes this a relatively cheap technology.
However, from this equation, it follows that carbon atom efficiency can never exceed
the value of 68%. Another disadvantage is that selectivities for saturated C4
-
C6
alkanes are low, because generally lower alkanes (<C4) are formed.
Typically, supported Pt catalysts are used. The nature of both the metal and the
support significantly influences the APR reactions of aqueous solutions of sugars with
C/O ratios of 1. As the acidity of the catalytic system increases, e.g., with a solid acid
catalyst support such as SiO
2
and Al
2
O
3
, the selectivity to alkanes increases due to the










































Search WWH ::

Custom Search