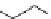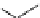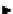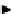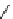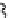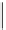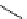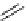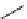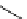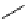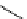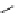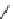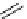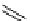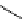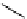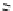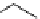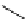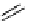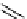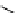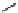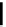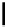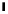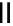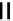Chemistry Reference
In-Depth Information
Then 150°C
O
K[
18
F]F-K
22
2
18
F
TsO
18
F
OTs
OTs
H
K
2
CO
3
, DMSO
90°C, 8 min
8 min
74
75
76
scheme 3.13
Two-step one-pot radiosynthesis of [
18
[18F]fluoroacetaldehyde (
76
) by the Kornblum oxidation of tosylate
75
by DMSO.
O
TBA[
18
F]F
O
SO
-
S
O
O
O
S
K
2
CO
3
, CH
3
CN
70°C, 5 min
S
18
F
O
O
O
O
77
78
H
N
R-NH
2
H
2
O / CH
3
CN
SO
-
R
SO
-
18
F
110°C, 15 min
79
, R=Et
80
, R=Lys
scheme 3.14
The use of two successive sulton ring opening reactions as radiolabelling step and bioconjugation step, resulting in
relatively polar products (
79
,
80
).
Kornblum oxidation of a tosylate by DMSO in the presence of K
2
CO
3
. Note that this reaction potentially can cause unwanted
precursor degradation in ordinary aliphatic radiofluorination in DMSO at temperatures ≥150°C.
Addition of a prosthetic group to a biomolecule is likely to cause an unwanted increase of lipophilicity. A way to counter
this can be the integration of a polyethyleneglycol (PEG) linker in the macromolecule/prosthetic group construct. An elegant
alternative has been proposed using the ring opening of a sulton by [
18
F]fluoride creating at the same time an anionic moiety
on the prosthetic agent. This increases the hydrophilicity and facilitates separation from the much less polar precursor [197]
(Scheme 3.14). Thus the
bis
-sulton
77
is radiofluorinated at one of the two sulton rings to give
78
, which is rapidly separated
from its precursor by a SPE method. The product
78
was then aminated with ethylamine or lysine by opening of the second
sulton ring. Products
79
and
80
could again be easily purified by SPE. A one-pot procedure without the intermediate purifi-
cation is also possible. This method should be extendable to macromolecule labelling.
3.5.2
prosthetic entities with fluorine-18 bound to aluminium, boron, or silicon
Radiometals play an important role in nuclear medicine imaging. Well-known examples are copper-64, gallium-68,
and yttrium-86 for PET and technetium-99m and indium-111 for SPECT. As far as macromolecules are concerned,
these metals are invariably attached in some oxidised form through a prosthetic polydental chelating entity [198]. This
labelling strategy is now also available for fluorine-18 by complexing the latter with aluminium as the Al[
18
F]F
2+
cation
[36, 199-204] (
81
, Figure 3.7). The chelates that have been used are of the NOTA or NODA type, and X-ray structure
determinations of the latter have been performed [203, 204]. The procedure is simple: The reaction takes place in
aqueous media and consists of adding AlCl
3
to the peptide-chelate adduct followed by the [
18
F]fluoride followed by
heating at 100°C for 15 minutes or less. Radiochemical yields of up to 85% have been reported [200], and the
well-known peptides octreotide [201] and RGD2 were successfully labelled and evaluated. This chemistry should lend
itself to a kit-like approach. Stereoisomers at the level of the orientation of the covalent Al-F bond in the complex seem
to be possible and separable with HPLC [200]. New NODA derivatives that function at lower temperatures for the
labelling step have recently been proposed [205].
-
O
2
C
S
N
NN
Peptide
2+
Al
18
F
N
H
H
N
CO
2
H
-
O
2
C
81
fIgure 3.7
An aluminium-fluorine-18 complex cation bound by a prosthetic NOTA ring.