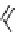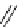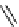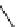Chemistry Reference
In-Depth Information
*F
OR
-
2OH
-
RB
+
3[
18
F]F
-
+
2H
2
O
RB
*F
+
2ROH
+
OR
82
*F
83
scheme 3.15
Tri[
18
F]luoroborate anion formation from the corresponding boronic esters
82
. The label in
83
is designated by *F and
not by
18
F to avoid the wrong impression that an individual molecule should necessarily bear three radioactive fluorine atoms at the time.
Boron is situated just above aluminium in the periodic table and shows similar binding properties to fluorine. However,
it is used in a different way than aluminium in radiofluorination [36], [206-213]. Arylboronic esters such as the pinacol
diester, but also free arylboronic acids, react at ambient temperature in aqueous media with fluoride to give anionic complexes
83
, in which all three available positions on the boron atom are occupied with fluorine atoms (Scheme 3.15), and which are
stable toward hydrolysis [207-209]. For labelling with fluorine-18, carrier fluoride is often added in order to make sure that
three fluorine atoms are introduced to each product molecule. The detrimental effect of this on the SRA is limited by the use
of as little precursor as possible in a small volume (a high concentration counteracts the relatively low rate constant of the
reaction) combined with the fact that three fluorine atoms enter each product molecule. With an excess of fluoride, the fluo-
rination efficiency can be quantitative [206]. The entry of three fluorine atoms per molecule on the one hand triples the initial
SRA but on the other hand may render the reaction less efficient when using no-carrier-added [
18
F]fluoride. However, even
with added carrier fluoride, SRAs of more than 1 Ci/µmol are attainable [207]. Boron-fluorine chemistry looks highly prom-
ising in prosthetic labelling because of its simplicity and its performance in aqueous media. Recent applications of this
methodology have been the labelling of an aryltrifluoroborate derivative of marimastate, a matrix metalloproteinase kinase
inhibitor, also in a kit-like procedure [211, 212], and a bodipy dye for dual PET and fluorescence imaging [213].
A third newcomer in prosthetic fluorine-18 labelling is silicon-fluorine bond formation [36, 210]. Although this bond,
like the B-F and the Al-F, is a strong one, it is relatively sensitive to hydrolysis in aqueous media. Bulky substituents on the
silicon atom effectively protect against this [214] and therefore, the prosthetic entity often takes the form of the aryl-
bis-
t
-butylsilanyl motive (
84
, Figure 3.8). Leaving groups that have been employed are OEt, OH, H, and F. The reaction can be
carried out under mild conditions and in aqueous media, although organic solvents such as MeCN or DMSO have been
applied more often thus far. Although unusual in fluorine-18 PET chemistry because of SRA considerations, the
18
F/
19
F
exchange on a fluoro-silicon precursor (
85
, X =F) has proved to be very useful. This exchange is very rapid and efficient,
probably proceeding through a penta coordinate intermediate. Therefore, very small amounts of precursor, down to 10 nmol
or less, are sufficient, which leads to SRAs high enough for receptor studies [215, 216].
p
-[
18
F]Fluorobenzaldehyde (
36
) is useful in both bioconjugation [186] and the radiosynthesis of smaller molecules [25],
and so is its silylated analogue
p
-(di-
tert
-butyl[
18
F]fluorosilyl)benzaldehyde (
86
), made by
18
F/
19
F exchange (Figure 3.8). It
can either be labelled as such and then conjugated to a peptide [215] by oxime formation, or it can be labelled when already
attached to the peptide [217, 218]. In the latter case, a kit-like approach should be possible. Similar prosthetic agents for
18
F/
19
F exchange, differing by having another functionality instead of aldehyde, have been used, that is, thiol [219, 220] (cou-
pled with maleimide derivated rat serum albumin (RSA) [220]), isothiocyanato (thiourea formation, RSA, apotransferrin,
bovine IgG [219, 221]), isocyanato,
N
-maleimido (with SH derivated RSA), carboxyl and its active esters [219], and azido-
methyl [222]. The silylated amino acid
p
-(di-
tert
-butylfluorosilyl)phenylalanine (
87
, Figure 3.8) was synthesised enantiose-
lectively recently, incorporated in Tyr3-octreoate derivatives, and labelled with fluorine-18 by
18
F/
19
F exchange [223]. The
bulky alkyl groups on the silicon atom contribute to an increase in lipophilicity of the biomolecule to be labelled; this may
be a problem. Therefore, a positively charged compound containing both the
p
-(di-
tert
-butyl[
18
F]fluorophenylsilane motive
and a tetraalkylammonium moiety was proposed as a lead compound for the development of a more hydrophilic prosthetic
group [224]. Another approach has been the insertion of a hydrophilic moiety in the peptide, for example, PEG [218]. Although
fluorine seems to be the preferred leaving group, H, OH, and OEt are also encountered [225-229]. Acid often accelerates the
t-Bu
t-Bu
t-Bu
O
XSi
Ar
18
F
18
F
Si
Si
t-Bu
H
t-Bu
t-Bu
CO
2
H
H
2
N
84
, X=
18
F
85
, precursor: X=OEt, OH, H, F
86
87
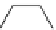
fIgure 3.8
General structure of most prosthetic [
18
[18F]fluorosilyl compounds (
84
) exemplified by
p
-(di-
tert
-butyl[
18
F]luorosilyl)benz-
aldehyde (
86
) and
p
-(di-
tert
-butylluorosilyl)phenylalanine (
87
), as well as general structure of their precursors (
85
).