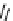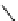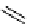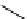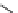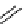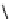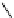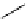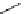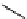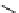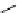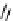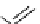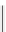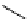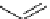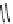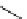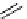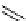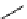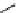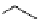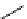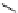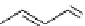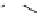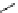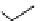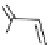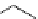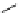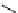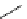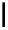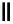Chemistry Reference
In-Depth Information
18
F
Cl
Cl
S
S
S
N
N
N
K[
18
F]F-K
222
+
K
2
CO
3
, DMSO
130°C, 10 min
18
F
F
CN
F
CN
CN
67
68
45 : 55
69
scheme 3.12
Radiolabelling of a high-affinity metabotropic glutamate receptor subtype 5 radioligand
68
from the corresponding
2-chloro-thiazole
67
. A radioactive side product (
69
) is formed by fluorine exchange on the benzene ring.
best leaving group of the halogens in aromatic substitution, which, for example, has been recently applied in the fluorination
of the aromatic amino acids L-DOPA, tyrosine, and phenylalanine [184].
3.5
labellIng of large bIologIcal molecules
3.5.1
the prosthetic group concept
When large biological molecules, such as peptides, proteins, antibodies, or oligonucleotides, are to be labelled with fluo-
rine-18, true labelling is not an issue because these molecules do not possess fluorine atoms of their own. If a polypeptide
structure contains one or more aromatic amino acid residues, it can in principle be labelled by an electrophilic H for
18
F
substitution [185], but this method is seldom used because of lack of specificity and side reactions. Instead, it is current prac-
tice to call on an intermediary chemical entity, a so-called prosthetic group, that can be, often specifically, attached to the
substrate molecule and also accommodate the fluorine-18 atom. Most frequently, the prosthetic agent is first labelled and
then attached to the macromolecule, so that the often harsh reaction conditions of radiofluorination do not need to be inflicted
upon the latter. Alternatively, the prosthetic entity can first be coupled to the macromolecule after which the adduct serves as
the labelling substrate. A large number of fluorine-18 prosthetic reagents have been published and have been compiled in a
recent review [186]. Figure 3.6 shows some representative examples: [
18
F]SFB (
70
), [
18
F]FBAM (
71
), [
18
F]FBnBrA (
72
),
and [
18
F]F-PEG-SH (
73
) [187-192]. The chemistry of the coupling between the prosthetic entity and the biomolecule is
varied and includes acylation,
S
-alkylation, oxime or hydrazone formation, click chemistry, photoconjugation, glycosylation,
amidation, amidination, and thiourea formation. Some more recent developments are discussed hereafter.
The prosthetic group carrying the fluorine-18 atom often has an appreciable size and usually is attached at a well-defined
position of the macromolecule, where it is thought not to perturb the latter's function, for example, at the terminal amino
group where it concerns a protein or peptide. However, random labelling of ε-amino groups of lysine residues in a polypep-
tide chain by acetaldehyde under reductive alkylation conditions is known to give minimal disturbance of the protein's struc-
ture and function because of the small size of the added ethyl group. [
11
C]Formaldehyde was used in the past to a similar end
in PET chemistry [193, 194]. Now [
18
F]fluoroacetaldehyde (
76
) has been used successfully for the labelling of recombinant
human interleukin-1 receptor antagonist, which contains nine lysine residues, in aqueous medium using sodium cyanoboro-
hydride as reducing agent [195]. The easy two-step one-pot preparation of [
18
F]fluoroacetaldehyde (
76
) is interesting and
worth a closer look (Scheme 3.13) [196]. 1-[
18
F]fluoro-tosyloxyethane (
75
) is produced in the habitual way (
74
, K[
18
F]F/
K
222
/K
2
CO
3
, MeCN, or DMSO, 90°C, 8 minutes). The MeCN is then evaporated and DMSO is added, followed by heating
at 150°C for 4 minutes and at 130°C for another 4 minutes while [
18
F]fluoroacetaldehyde (
76
) is distilling out. This is the
O
O
O
O
N
Br
N
O
H
O
N
O
6
18
F
SH
O
O
3
18
F
18
F
18
F
70
, [
18
F]SFB
71
, [
18
F]FBAM
72
, [
18
F]FBnBrA
73
, [
18
F]F-PEG-SH
fIgure 3.6
Some representative examples of [
18
F]prosthetic reagents (
70
‒
73
).