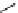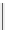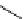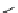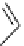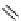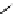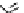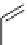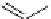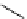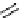Chemistry Reference
In-Depth Information
K[
18
F]F-K
222
K
2
CO
3
, DMSO
145°C, 2 min
TFA
N
H
O
N
Boc
O
N
Boc
O
N
N
N
CH
2
Cl
2
RT, <1 min
H
3
C
H
3
C
18
F
N
+
CH
3
18
F
or
54
microwave activation
100 W, 1 min
55
(
not isolated
)
CF
3
SO
-
56
, [
18
F]F-A-85380
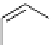
scheme 3.11
Synthesis of the nicotinic acetylcholine receptor ligand [
18
F]F-A-85380 (
56
) from the Me
3
N
+
-pyridine precursor
54
, via
non-isolated
55
as an example of a heteroaromatic radiofluorination.
O
58
, [
18
F]FPyME
R
H
3
C
O
H
18
F
N
O
N
N
N
N
O
OCH
3
18
F
N
O
18
F
n
O
n
61
, [
18
F]FEP (R=H, n=1)
62
, [
18
F]FPhEP (R= Ph, n=1)
63
, [
18
F]F
2
PhEP (R=FPh, n=1)
H
18
F
N
59
, [
18
F]FPyKYNE (n=2)
60
, [
18
F]FPy5yne (n=3)
57, 6
-[
18
F]uoro-PBR28
64
, [
18
F]NCFHEP (R=H, n=2)
18
F
N
N
N
t-Bu
NH
2
N
18
F
O
N
N
N
HO
O
HO
OH
NEt
2
66
, [
18
F]PBR132
65
, [
18
[18F]fludarabine
fIgure 3.5
various radioligands (
57
,
61
‒
66
) and prosthetic reagents (
58
‒
60
) as examples of radiolabelled compounds obtained by
heteroaromatic radiofluorination.
(Figure 3.5). The activating effect by the nitrogen atom is hardly perturbed by the presence of deactivating groups such
as methoxy or methyl
ortho
to the leaving group [165, 173]. This is also reflected in the syntheses of the prosthetic agents
[
18
F]FPyKyNE (
59
) [174], [
18
F]FPy5yne (
60
) [175, 176], and [
18
F]FPyMe (
58
) [177].
The scope of the radiofluorination of pyridine itself was studied; it was found that nitro and trimethylammonium groups
perform better than the halogens [178], that the α position is somewhat more reactive than the γ position, and that the β posi-
tion is completely unreactive [179]. However, in analogy to the above example of
meta
-[
18
F]fluorobenzaldehyde (
48
),
meta
-
[
18
F]fluoropyridine can be produced from an appropriate iodonium salt [152]. The β-position is the most stable one for
in
vivo
defluorination, and the γ position is the most labile, in fact 4-[
18
F]fluoropyridine rapidly hydrolyses in water to 4-[
18
F]
fluoro-2-pyridone. The α-position is practically always chosen for radiofluorination because the chemistry is the easiest and
the fluorine in this position is relatively stable
in vivo
. In aromatic substitutions, fluorine is the best leaving group of the hal-
ogens, and indeed
19
F/
18
F exchange with 2-fluoropyridines is very easy with high yields using small substrate amounts,
resulting in very reasonable SRAs in the order of 10 GBq/µmol [173]. Examples of radiofluorination of heteroaromatic sys-
tems with two nitrogen atoms in the ring are [
18
F]fludarabine (
65
) [180] (2-position of a pyrimidine moiety) and [
18
F]
PBR132 (
66
) [181] (3-position of a pyridazine moiety).
2-Chloro- and 2-bromo-1,3-thiazoles were recently shown to react easily with [
18
F]fluoride in DMSO to give the
corresponding 2-[
18
F]fluoro-1,3-thiazoles [182]. Their reactivity is comparable to those of 2-chloro- and 2-bromopyridine.
The 2-[
18
F]fluoro-1,3-thiazole moiety presents a potential alternative to the 2-[
18
F]fluoropyridine entity in prosthetic group
design. In contrast to the 1,3-thiazole ring itself, which is susceptible to oxidative ring opening
in vivo
, the 2-fluoro-1,3-
thiazole ring is metabolically much more stable. A first application has been the synthesis of a high-affinity metabotropic
glutamate receptor subtype 5 radioligand (
68
) [183] (Scheme 3.12). Note that the fluorine atom on the benzene ring is sus-
ceptible to considerable exchange giving rise to
69
, in spite of its non-activated position, a reminder of the fact that fluorine is the