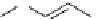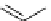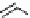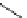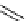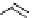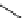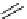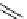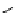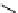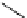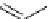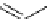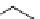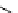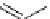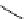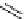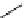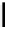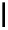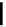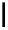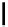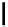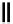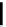Chemistry Reference
In-Depth Information
Br
-
Cs[
18
F]F
CsHCO
3
, TEMPO
18
F
+
18
F
I
+
DMF
MW (110°C), 5 min
47
48
49
OH
H
O
scheme 3.9
Synthesis of
meta
-[
18
F]luorobenzaldehyde (
48
) via an iodonium precursor
47
.
CH
3
CH
3
51
4-component
reaction
EtOH
O
N
NH
2
N
NC
H
O
HO
2
C
OH
100°C, 30 min
62%
50
52
53
18
F
36
18
F
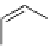
scheme 3.10
A convergent synthesis involving four components (
36
,
50
,
51
, and
52
) applied to the radiosynthesis with fluorine-18 of
53
.
which probably failed because of oxidation of the methylene group of
42
under the fluorination conditions. Therefore, an
alternative 4-(4-nitrobenzoyl)piperidinyl precursor
44
was chosen. The activating benzoyl carbonyl group in
45
is then
reduced after the fluorination. With an activating group at a
meta
position, the radiochemical yields are considerably lower,
but sometimes useful activities can still be obtained especially with microwave heating [127, 140-143].
Meta
-[
18
F]fluorobenzaldehyde (
48
) was recently made from an appropriate diaryliodonium precursor
47
(Scheme 3.9) [144]
with a yield of 80%. It was subsequently converted into
meta
-[
18
F]fluorobenzyl bromide, which was needed for the synthesis
of a labelled tyrosine kinase inhibitor containing a
meta
-[
18
F]fluorobenzylether moiety [145]. The leaving group in this posi-
tively charged precursor
47
is an aryl iodide, the incoming [
18
F]fluoride being predominantly directed toward the most electron
deficient aryl system, in this case the benzene ring bearing the formyl group, leading to
48
rather than to
49
. This orientation
was reinforced in the presence of a radical scavenger, and the yield was also dependent on the choice of the counter ion.
This reaction type provides a general strategy for the radiofluorination of arenes, especially the electron-rich ones, which are
otherwise not accessible with habitual procedures of aromatic nucleophilic fluorination, but also electron-deficient ones as in
the above example [146-159]. Apart from electronic effects of the substituents, a steric effect also exists, named the
ortho
effect,
which implies that an
ortho
substituent on one of the rings, for example, a methyl group, directs the fluorination toward that ring
[146, 147, 155, 156]. Aromatic moieties other than benzenes, such as 2-thienyl, have been used to direct the fluorination to the
other aromatic ring [154, 157]. Although the number of published applications is slowly growing, the use of the method remains
modest because of complicated precursor syntheses that do not always lead to very stable compounds.
Multicomponent chemistry is a convergent synthetic approach in which three or more substrates react simultaneously in
one step. This could be a very useful tool in radiofluorination in order to access products with the fluorine label in positions
that would normally not be considered as feasible. In a first validation of this concept, the easily accessible
para
-[
18
F]fluo-
roacetaldehyde (
36
) and substituted derivatives as well as
para
-[
18
F]fluorobenzoic acid were condensed in four different
types of convergent three- or four-component reactions, optimised for the specific requirements of stoichiometry and time
in PET chemistry, leading to labelled compounds that would not be easily accessible via traditional late-stage bimolecular
condensation involving a [
18
F]fluoroaromatic compound or [
18
F]fluoride itself [160] (Scheme 3.10). A multicomponent ver-
sion of fluoroalkylations of aromatic compounds was also proposed lately [161]. A multicomponent
N
-[
18
F]fluoroalkylation
has been shown useful in the synthesis of [
18
F]fluoroethylcholine [162].
3.4.2.3 Nucleophilic Heteroaromatic Substitution
Scheme 3.11 depicts the synthesis of the nicotinic acetylcholine
receptor ligand [
18
F]F-A-85380 (
56
) [163], which is a heteroaromatic radiofluorination. When an aromatic ring contains one
or more heteroatoms, nucleophilic radiofluorination is enhanced relative to the homoaromatic counterpart [67, 164]. Most
important is the pyridine ring, in which the ring nitrogen exerts a similar, if not slightly greater activating effect on the α and
γ positions as the aldehyde group in benzaldehyde [165].
No further activating groups on the pyridine ring are needed to make the reaction proceed, as can also be noted in the syn-
theses of the TSPO [166], ligand 6[
18
F]fluoro-PBR28 (
57
) [167, 168], and the [
18
F]epibatidine derivatives
61‒64
[169-172]