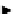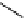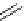Chemistry Reference
In-Depth Information
reputed for deactivation of fluoride in the same way as water [52, 110-117]. Most information on this method is about primary
mesylates. Sometimes the yields are better than in aprotic solvents, and elimination reactions are suppressed [52, 114]. It is
believed that the tertiary alcohols solvate the [
18
F]fluoride in a much less tight way than water does, so that nucleophilicity
is not inhibited. Also they may enhance the nucleofugacity of the sulphonate leaving group by hydrogen bonds to the latter's
oxygen atoms [118]. A comparative study on benzylic chloride and mesylate model compounds showed that tertiary alcohols
perform less well than MeCN and DMF for this reaction. The same study also confirmed the current opinion that they do not
work for aromatic substitution [118], although a heteroaromatic substitution in
t
-butanol has been published [119]. The
important radiopharmaceutical [
18
F]FLT (
3
) has been produced in
t
-butanol, but the question whether elimination, notorious
for this molecule [120], had been suppressed or not was not addressed [111, 113].
Ionic liquids are salt-like compounds constituted by an organic cation like 1-butyl-3-methylimidazolium (bmim) and an
anion that can be inorganic (e.g., BF
4
-
or SbF
6
-
) or organic, such as a sulphonate. They are in the liquid state at ambient tem-
perature and can serve as solvents, usually with a co-solvent (MeCN), in nucleophilic aliphatic radiofluorination [121-125].
Large amounts of water are tolerated, offering the advantage that a drying procedure of the [
18
F]fluoride is not necessary. The
presence of water seems to reduce elimination, for example, in 1-mesyloxy-2-(naphth-2-yl)ethane, a substrate that normally
is very susceptible to elimination by fluoride [121, 122]. [
18
F]FDG (
2
) and [
18
F]FLT (
3
) have been made successfully in ionic
liquid systems [124, 125]. The use of
t
-butanol as a co-solvent in an ionic liquid does not seem advantageous; [121] however,
incorporation of a tertiary alcohol moiety in the organic cation of an ionic liquid may produce a synergistic effect [126].
3.4.2.2 Nucleophilic Homoaromatic Substitution
Homoaromatic nucleophilic radiofluorination is in general more difficult
than its aliphatic counterpart, and higher temperatures are usually required. The solvent should be polar and aprotic, like MeCN,
DMSO, or DMF, and no divergences to more aqueous systems, as for the aliphatic case above, have been reported. A represen-
tative example is shown in Scheme 3.6.
While the aliphatic substitution has a one-step mechanism with a single transition state (
38
, Scheme 3.7b), the aromatic
substitution passes via a normally negatively charged intermediate like
35
(Scheme 3.7a), the so-called Meisenheimer com-
plex, resulting from the addition of the [
18
F]fluoride anion to the substrate molecule, in this case
34
.
The formation of the Meisenheimer complex
35
is the rate-determining step. The subsequent loss of the leaving group is
fast. The most current leaving groups used in this reaction are the nitro group, the halogens (reactivity order: F > > Cl > Br
>> > I, which is the inverse of aliphatic reactions [127]), and the trimethylammonium group. The latter has the particular
O
O
K[
18
F]F-K
222
K
2
CO
3
, DMSO
180°C, 30 min
O
O
N
N
18
F
N
O
2
N
N
S
S
N
H
3
C
N
H
3
C
33
, [
18
F]setoperone
32
scheme 3.6
Representative example of a homoaromatic radiofluorination. Synthesis of the serotoninergic 5-HT
2
ligand [
18
F]setoperone (
33
).
Meisenheimer
complex
(a)
HO
HO
HO
K[
18
F]F-K
222
K
2
CO
3
, DMSO
MW (40W), 25 min
18
F
18
F
NO
2
NO
2
18
F
-
34
35
36
Transition state
(b)
-
LG
LG
-LG
-
18
F
-
R
3
R
2
R
1
R
3
R
3
R
1
R
1
R
2
R
2
18
F
18
F
37
38
39
scheme 3.7
(a) Two-step reaction mechanism of an aromatic radiofluorination as illustrated by the synthesis of
p
-[
18
F]luorobenzaldehyde (
36
),
showing the intermediate Meisenheimer complex
35
. (b) One-step reaction mechanism of an aliphatic radiofluorination (from
37
to
39
via
38
).