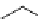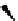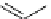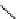Chemistry Reference
In-Depth Information
R
K[
18
F]F (without K
222
)
K
2
CO
3
, CH
3
CN
O
O
O
S
18
F
O
CH
3
O
n
MW 90W
5×2 min, 130°C
R
O
28
29
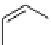
scheme 3.4
Synthesis of a fluorine-18-labelled aliphatic compound
29
by the use of a nucleophilic assisting leaving group. Compound
28
complexes by its PEG arm the counter cation, positioning the latter so that it can stabilize the transition state.
1) K[
18
F]F-K
222
K
2
CO
3
, CH
3
CN
90°C, 10 min
F
F
FF
FF
FF
18
F
O
O
F
F
S
O
FF
FF
FF
F
Then
FSPE-purication
and
2) aq. 2M CF
3
SO
3
H
90°C, 10 min
CO
2
H
H
CO
2
CH
3
N
31
, cis-4-[
18
F]uoro-L-proline
30
Boc
scheme 3.5
Synthesis of
cis
-4-[
18
F]luoro-L-proline (
31
) from a polyfluorinated sulphonate precursor
30
.
A new concept is represented by the so-called nucleophile assisting leaving groups (NALGs). These are primary arene-
sulphonates that bear at the
ortho
position an entity that has chelating properties toward metal cations, for example, a PEG
motif (
28
, Scheme 3.4). While the [
18
F]fluoride attacks the sulphonate-bearing aliphatic carbon, its accompanying metal
cation is complexed, allowing it to stabilise the negative charges on both the incoming fluoride and the leaving sulphonate
unit during the transition state. No phase transfer kryptand is needed, and the rate of the reaction is considerably enhanced
relative to the corresponding tosylate under the same reaction conditions [98].
The application of sulphonate esters having a long polyfluorinated alkyl chain attached to the sulphur atom is a new and
remarkable trend [99]. These sulphonates make very good leaving groups because of the electron-attractive power of the fluo-
rine atoms. More important even is the fact that the polyfluorinated state of the starting material facilitates separation from the
non-polyfluorinated product by so-called fluorous solid-phase extraction (FSPE) based on the high mutual affinity between a
polyfluorinated compound and a fluorous solid phase [100]. Such a separation could potentially replace an HPLC purification.
For example,
cis
-4-[
18
F]fluoro-L-proline (
31
) was synthesised from the corresponding 1H,1H,2H,2H-perfluorodecane-1-
sulphonate precursor (
30
) and purified by FSPE in somewhat higher yields than with the tosylate (Scheme 3.5) [101]. Note
that the first two carbon atoms of the chain are not fluorinated. Indeed, in model studies benzyl perfluorobutanesulphonate or
-perfluorooctanesulphonate were reported not to give the desired product [99]. Some evidence was recently presented pointing
to the possibility of an attack by fluoride on the sulphur atom, provoking expulsion of perfluoroalkene having eliminated a
fluoride ion [102]. On the other hand, the
in situ
prepared perfluorobutanesulphonyl precursor of
cis
-4-[
18
F]fluoro-L-proline
(
31
) was reported to give the desired product [103, 104].
Polyfluorinated long-chain sulphonate precursors for [
18
F]FDG have been attached to a resin with the chain acting as a linker
[105, 106]. The leaving group and also unreacted or modified precursor remain on the resin, which facilitates purification. The
presence of the fluorine atoms greatly enhances the reactivity, which was higher than that of the corresponding triflate. Leaching
of non-radioactive fluoride from the chain, for example, by exchange with [
18
F]fluoride, was claimed to be negligible. The issue
of
18
F/
19
F exchange is something to be kept in mind when the SRA is important because the latter is reduced by this phenomenon.
While there does not seem to be any exchange with compounds such as perfluoro-
n
-hexane or perfluoro-
n
-hexylbenzene
[107] and only a little bit in triflates [108], it is very important in tresylates (CF
3
CH
2
SO
3
R) in which
18
F/
19
F exchange seriously
competes with nucleophilic displacement of the tresylate group [86, 109]. It was reported that nucleophilic radiofluorination
with 1H,1H,2H,2H-perfluorooctanesulphonates resulted in a ten- to hundred-fold decrease in SRA [101].
Solvents
The solvents applied in both aliphatic and aromatic radiofluorination are usually of the polar aprotic kind, such
as MeCN, DMSO, or DMF. Although these solvents may not be optimal in terms of relative stabilisation of transition state
and starting products, they have the merit of promoting solubility of the fluoride and the precursor and not presenting loose
protons that can deactivate the [
18
F]fluoride anion. This last concept has lately been moderated by two developments in
aliphatic radiofluorination, namely bulky alcohols and ionic liquids. It was shown that bulky tertiary alcohols like
t
-butanol
or
t
-amyl alcohol can act as solvents in nucleophilic aliphatic radiofluorination, in spite of the hydroxy group, generally