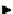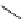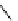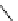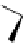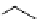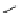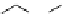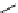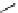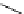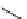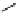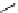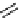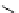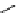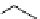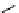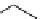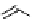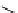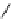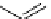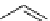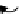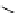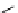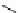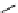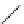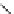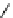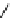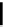Chemistry Reference
In-Depth Information
OH
OAc
K[
18
F] F-K
222
K
2
CO
3
OAc
OMs
aq. 1N HCl
Reux
15 min
O
O
O
HO
HO
AcO
AcO
AcO
AcO
OAc
OAc
OH
CH
3
CN
reux, 5 min
18
F
18
F
2
, [
18
F]FDG
18
19
O
O
O
CH
3
H
CH
3
H
CH
3
N
N
N
K[
18
F]F-K
222
KHCO
3
aq. 1N HCl
50°C, 10 min
N
O
N
O
N
O
DMTrO
DMTrO
HO
O
O
O
DMSO
160°C, 10 min
18
F
18
F
3
, [
18
F]FLT
20
21
scheme 3.2
Radiosynthesis of [
18
F]FDG (
2
) and [
18
F]FLT (
3
) illustrating inversion of configuration in these secondary SN2 substitutions
(from precursor
18
(respectively
20
) to intermediate
19
(respectively
21
)).
O
O
Boc
CH
3
CH
3
N
1) K[
18
F]F-K
222
K
2
CO
3
, CH
3
CN
80°C, 20 min
2) HCl / MeOH
80°C, 10 min
HN
O
N
N
O
18
F
THPO
HO
O
O
23
, [
18
F]FMAU
22
THPO
OMs
OH
scheme 3.3
Two-step synthesis of [
18
F]FMAu (
23
) with the pyrimidine moiety in place, replacing the habitual three-step procedure,
which included final [
18
F]sugar pyrimidine coupling.
H
O
CH
3
N
18
F
-
BnOCO
N
N
25
CH
3
O
N
+
18
F
-
Bz
N
18
F
-
24
26
27
18
F
-
O
fIgure 3.3
Radiofluorination by ring-opening reactions.
labelled product with the
18
F in the 'down' position. The yields were very low, but possibly worthwhile because the alternative
is the time-consuming three-step procedure, habitual for this type of molecule, in which the pyrimidine moiety is attached
after the fluorine-18 introduction. The low yield is probably due to steric hindrance. Another reason could be a side reaction,
common in this type of chemistry, which is the elimination of methylsulphonic acid by base attack, including that by [
18
F]
fluoride, on the hydrogen at the 3′-position, which also deactivates the [
18
F]fluoride. Note that corresponding purines
with the more reactive triflate as leaving group have given reasonable yields, although here the 2′-position seems sterically
hindered to some extent [71].
The halogens chlorine [72, 73], bromine [10, 74, 75], and iodine [76-79] are also used as leaving groups but less than
the sulphonates. Exchange reactions with aliphatic fluorine have only been reported with polyfluorinated carbon centres
[80-86]. Aliphatic radiofluorination based on ring opening, in which the leaving group remains attached to the molecule, [23,
87-94] remains a topical subject. The examples shown in Figure 3.3 feature the regioselective opening of an epoxide ring
(
24
) to give 4-(3-[
18
F]fluoro-2-hydroxypropoxy)benzaldehyde for peptide labelling [95], the opening of 2-methylaziridine
derivatives (
25
and
26
) regiocontrolled by the choice of the
N
-protective group [96], and the ring opening of an azetidinium
salt
27
to give a 2-[
18
F]fluoroethylpiperazine [97].