Chemistry Reference
In-Depth Information
Since the initial reports of the two types of tetrazine diels-Alder reactions in bioconjugation, there have been many reports
on the development and biomedical applications of the reactions [176], such as
in vitro
labelling of quantum dots [177] and
nucleic acids [178, 179], modification of polymer [180], labelling of live cell surface antigens using small dye molecules or
nanoparticles [172, 174, 181], imaging of intracellular small molecules [173, 182], and
in vivo
imaging [183-186]. The first
application of the tetrazine-
trans
-cyclooctene ligation for
in vivo
imaging was performed using a pretargeted labelling protocol
(similar to Scheme 2.9) [183]. Anti-TAg72 (TAg72 - a biomarker overexpressed in a wide range of solid tumours) monoclonal
antibody CC49 was first tethered to
trans
-cyclooctene, and doTA was conjugated with the Fox type tetrazine. In a PbS buffer,
the cycloaddition finished within 3 minutes at 1.67 μM and the second-order rate constant was found to be 13090 M
-1
s
-1
at 37°C,
very promising for
in vivo
labelling. Faster kinetics than the original report by Fox were expected, because a large increase of
the reaction rate in aqueous media was seen in many other cases. Very low nonspecific binding of doTA(In-111)-tetrazine
conjugate to unmodified CC49 or other media constituents was observed, and the probes showed decent stability in live mice
(75% remained after 24 hrs).
In vivo
administration of the
trans
-cyclooctene-modified CC49, followed one day later with injec-
tion of doTA(In-111)-tetrazine, resulted in significant localisation of radioactivity in the tumour, as imaged by SPeCT/CT of
live mice three hours later. only a background signal was observed in tumours in the mice administered with unmodified CC49
and doTA(In-111)-tetrazine or with
trans
-cyclooctene-modified nonspecific antibody to TAg72 and doTA(In-111)-tetrazine.
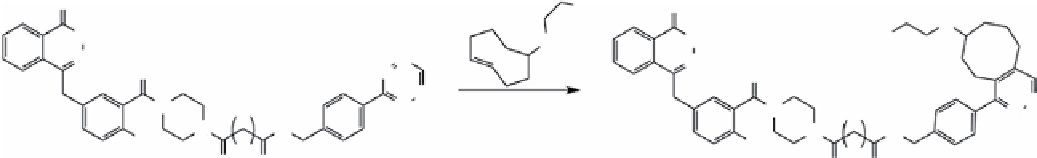
The Fox group verified the applicability of the tetrazine-
trans
-cyclooctene ligation to introduce an F-18 label [187].
Weissleder and co-workers took it one step further and modified inhibitor AZd2281 [188] of poly-AdP-ribose-polymerase
1(PARP-1) with F-18 (Scheme 2.11) [189]. This is advantageous because direct F-18 labelling of the AZd2281 is challeng-
ing and usually leads to low radiochemical yields and purity. F-18-AZd2281 showed targeted accumulation in mice with
breast cancer tumour xenografts that overexpressed PARP-1 [184]. using the tetrazine-
trans
-cyclooctene ligation, F-18 was
also attached to Rgd peptide with high efficiency (5 min > 90% yield). The F-18-Rgd conjugate showed superior tumour
uptake
in vivo
, with specificity for α
v
β
3
integrin [185]. Tetrazine-norbornene cycloaddition was used to construct antibody-
Cu-64 or antibody-Zr-89 conjugates
in vitro
by tethering anti-HeR2-antibody and chelator (doTA or dFo) to norbornene
and tetrazine respectively followed by the cycloaddition and radiometallation [186].
In vivo
PeT imaging and biodistribution
studies showed significant and specific uptake of the conjugates in HeR2-positive xenografts with little background noise.
2.2.3.2 Radical Thiol-Ene Reaction
Reactions between alkenes and thiols can occur either through free-radical addition
of thiol to alkenes (termed thiol-ene reaction) or through Michael addition of thiol to electrophilic carbon-carbon double bonds
(Scheme 2.12). While application of the Michael addition has been explored widely in the past, here we will only focus on the
radical type thiol-ene reaction for its rapid advances in recent years [24].
Thiol-ene reactions are considered to be a type of 'click chemistry,' owing to the high reactivity of the reactants upon
addition of low concentrations of catalysts with or without uV irradiation. Their high yields within short periods under ambient
conditions and readily installable thiols or alkenes to various molecules make them one of the most frequently used reactions
in polymer sciences, such as modification of substrate surface, fabrication of photolithography and microdevices, and formation
of nanostructured networks [190]. nearly all sterically favourable alkenes can undergo thiol-ene reactions, while electron-rich
(vinyl) and strain-involved alkenes (norbornene) react faster than electron-poor alkenes.
Recently, thiol-ene chemistry has been explored as a conjugation method to modify various molecules, such as polymers
[191, 192], dendrimers [193, 194], peptides or proteins [195], and nanoparticles [196-198]. The robustness of thiol-ene
reactions was demonstrated in the synthesis of a fourth-generation dendrimer (g4); all of the 48 peripheral alkene groups
could be modified nearly quantitatively in 30 minutes using the photoinitiator 2,2-dimethoxy-2-phenylacetophenone
(dMPA) under uV irradiation (Scheme 2.13) [194].
O
O
18
F
O
18
F
NH
N
NH
N
O
N
O
N
O
N
3 minutes
60% radiochemical yield
96% radiochemical purity
N
N
N
N
N
H
H
N
N
N
N
F
F
3
3
O
O
O
O
scheme 2.11
F-18 labelling of PARP-1 inhibitor AZd2281 using tetrazine-
trans
-cyclooctene ligation.
H
R′
a) free radical
b) Michael addition
S
R-SH
+
C=C
R
R′
H
H
scheme 2.12
Thiol-ene coupling via either free radical (a) or Michael addition reactions (b).