Chemistry Reference
In-Depth Information
tetrazine-
trans
-cyclooctene reaction as a ligation method can be very practical in the sense that the starting tetrazine
derivative can be synthesised in several steps from commercially available materials, and
trans
-cyclooctene derivative can
be generated from its
cis
-cyclooctene analogue using a one-step photochemical reaction reported by the Fox group [171].
Weissleder and co-workers discovered a more stable monosubstituted tetrazine derivative for biomedical application,
after noticing some degradation of 3,6-di-(2-pyridyl)-s-tetrazine (Scheme 2.8). Importantly, they found that a dye-labelled
tetrazine showed even faster kinetics while reacting with
trans
-cyclooctene modified antibody, at a second order rate constant
of 6000 M
-1
s
-1
at 37°C. The utility of the reaction for live-cell labelling and in serum-containing media was demonstrated
using a pre-targeted labelling protocol. A549 lung cancer cells overexpressing egFR were pretargeted by anti-egFR anti-
body cetuximab labelled with
trans
-cyclooctene and rhodamine, followed by subsequent ligation with a nIR dye (VT680)
labelled tetrazine. efficient coupling was achieved within 30 min with a submicromolar tetrazine probe, which co-localised
with the rhodamine signal, showing the specificity of the tetrazine-probe for
trans
-cyclooctene tethered to an antibody
(Scheme 2.9). Interestingly, the extent of the reaction is proportional to the number of
trans
-cyclooctenes on the antibody,
as shown by flow cytometry and confocal imaging. Similarly, magnetofluorescent nanoparticles attached to tetrazine could
also be introduced to live cell surface using the pretargeted labelling protocol [172]. Compared to the traditional preas-
sembled antibody-nanoparticle approach, there are several obvious advantages: It is easier to conjugate antibodies with
trans
-cyclooctene than with nanoparticles; maximisation of the nanoparticles on an antibody is possible, while higher load-
ing of nanomaterials on antibodies tends to lead to aggregates and precipitates; an antibody binds to cell surface antigens
before the conjugation with nanoparticles, decreasing the chances of blocked binding sites by bulky nanoparticles. This
antibody pretargeted labelling protocol was also used to label intracellular molecules [173].
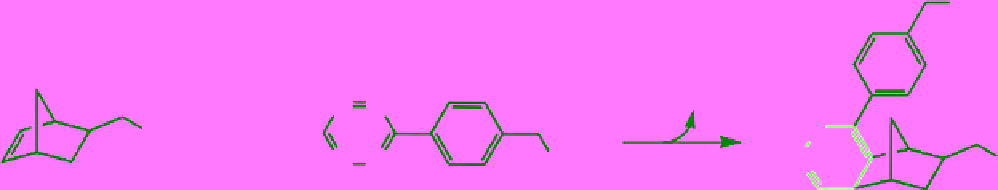
Weissleder and co-workers also explored the reaction between tetrazine and norbornene as a new bioconjugation strategy
(Scheme 2.10) [174]. To solve the stability issue of tetrazines in water [175], they incorporated a benzylamine into tetrazine,
making it very stable in a buffer and moderately stable in serum. Although the kinetics of this reaction was not as good as
the
trans
-cyclooctene-tetrazine ligation, it showed a second-order rate constant of 1.9 M
-1
s
-1
and 1.6 M
-1
s
-1
in an aqueous
buffer and serum respectively. The reaction was sufficiently fast in live cell imaging using the aforementioned pretargeted
labelling protocol. efficient coupling was achieved within 30 minutes with the tetrazine probe at the micromolar scale, and the
tetrazine-probe showed specificity for norbornene attached on antibody.
Probe
O
HN
O
O
N
N
N
N
Diels-Alder reaction
mAb targeting
Cell
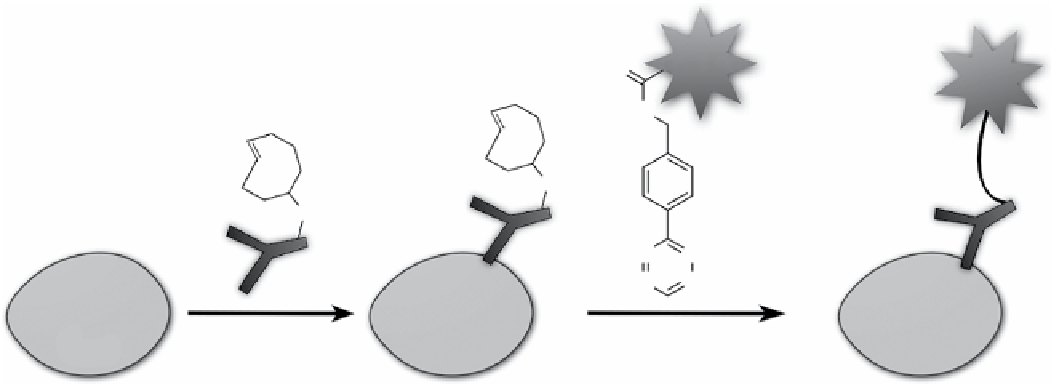
scheme 2.9
Live cell pre-targeting. Step one,
trans
-cyclooctene-modiied monoclonal anti-egFR antibody was targeted to egFR
on cell surface. Step two, the pre-targeted cells were labelled with tetrazine-modified fluorophores or other imaging probes.
NH
2
N
2
NN
HN
N
CO
2
H
+
Solvent
NH
2
NN
CO
2
H
scheme 2.10
Conjugation reaction between tetrazine and norbornene.