Chemistry Reference
In-Depth Information
O
N
O
N
3
O
O
O
O
OGDP
HO
HO
O
O
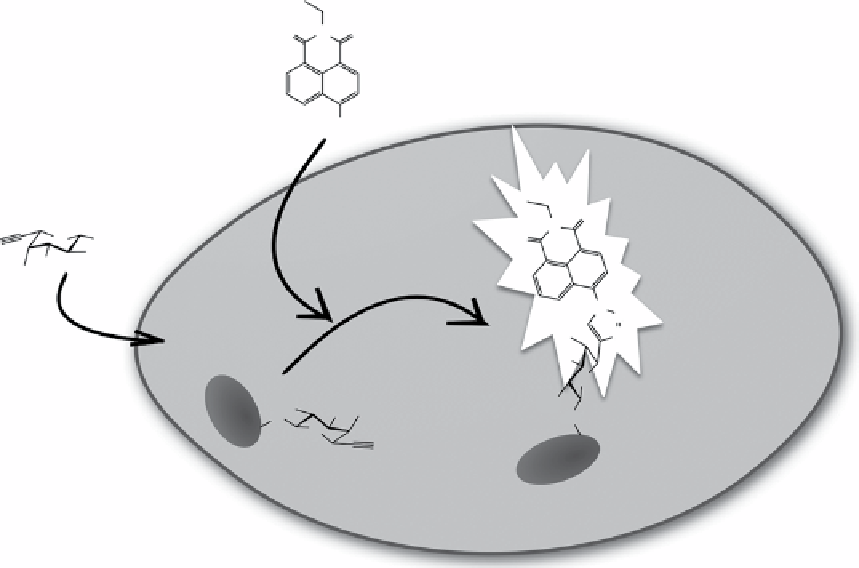
fIgure 2.4
Fluorescent labelling of fucosylated glycans in cells via CuAAC. Propargyl or azide-modified fucoside was first incorporated
to proteins inside cells via glycosylation pathways, and fucosylated proteins could then be imaged using azide or propargyl-modified fluoro-
genic probe 1,8-naphthalimide derivative, which turned on upon reaction with modified fucosylated proteins.
sometimes at slightly elevated temperatures [74]. However, the high concentration of copper ion used in the reactions becomes
a concern in causing adverse side reactions or toxicity in living systems [133], therefore limiting the usage of CuAAC in living
cells or organisms. nonetheless, a few groups have reported the use of regular CuAAC in fixed mammalian cells. Wong and
co-workers applied the reaction to probe fucosylated glycans in cells [134]. Fucosylation sites could be identified after incor-
poration of alkyne or azide modified gdP-L-fucose to glycoproteins by the addition of an azide or alkyne modified cell-pene-
trating fluorogenic probe in the presence of millimolar CuSo
4
and ascorbate (Figure 2.4).
In one report by Jao et al
.
, CuAAC was performed in cells to image RnA transcription and turnover [135]. Incorporation
of alkyne-modified uridine into newly transcribed RnA can be visualised by treating the cells with fluorescent azides.
dynamic labelling of dnA was achieved using alkyne-modified deoxyuridine [136]. Jao et al. also demonstrated the use of
CuAAC in imaging incorporation of propargylcholine, an analogue of the most abundant head group choline, into phospho-
lipids [137]. In another example, cells were metabolically labelled with either alkyne or azides (using artificial amino
acids or carbohydrate) on the cell surface, and semiconducting polymer dots were then attached to cell surface through
CuAAC using millimolar CuSo
4
and ascorbate [138].
Recently, after screening of a small library of analogues of TbTA, a commonly used ligand to assist CuAAC, Wu and
coworkers have identified one tighter binding ligand, therein termed as bTTeS, which required only micromolar copper for
efficient labelling in living cells within minutes, without apparent toxicity after days (Figure 2.3) [67]. using the CuAAC-
bTTeS system,
in vivo
imaging of fucosylated glycans during zebrafish early embryogenesis was achieved for the first time.
This approach may open a new era for
in vivo
imaging of living systems using CuAAC, which was not possible to achieve
previously.
2.2.2.3 Cyclooctyne-Azide Cycloaddition
In order to render the azide-alkyne cycloaddition biocompatible inside cells
without disturbing normal cellular functions, efforts have been made to optimise the reaction. Ju and co-workers found use of
electron-deficient internal or terminal alkynes allowed the azide-alkyne cycloaddition to complete under biological conditions
in hours without any catalysts, such as copper [139]. However, under the same conditions, no reaction occurred for the alkynes
without any neighbouring electron-withdrawing group. Although not perfect, this approach made it promising to achieve bio-
compatible azide-alkyne cycloaddition.
besides tuning the electronic effects, release of strain energy can be an alternative approach. Inspired by the discovery by
Wittig and Krebs that cyclooctyne, the smallest of the stable cycloalkynes, and phenyl azide can undergo explosion-like