Chemistry Reference
In-Depth Information
+
CuSO
4
(1 mol%), NaAsc (5 mol%)
H
2
O:tBuOH (2:1), r.t. 8 h
N
N
-
N
O
N
N
N
+
O
scheme 2.5
Copper (I)-catalysed azide-alkyne cycloaddition (CuAAC) to give only 1,4-isomer.
NH
N
N
N
N
NH
N
N
Diisopropylethylamine
(DIPEA)
Tetramethyl ethylene diamine
(TEMED)
1S,2S-bis (methylamino)hexane
(BMAH)
Terpyridine
N
N
KOOC
N
N
N
N
N
N
N
N
N
N
N
N
N
COOK
N
N
N
N
N
N
N
N
N
OSO
-
NN
N
NN
BTTES
NaO
3
S
SO
3
Na
KOOC
Tri(1-benzyle-[1,2,3]-triazol-4-ylmethyl)
amine
(TBTA)
Bathophenanthroline disulfonate
(Batho)
(BimC
4
A)
3
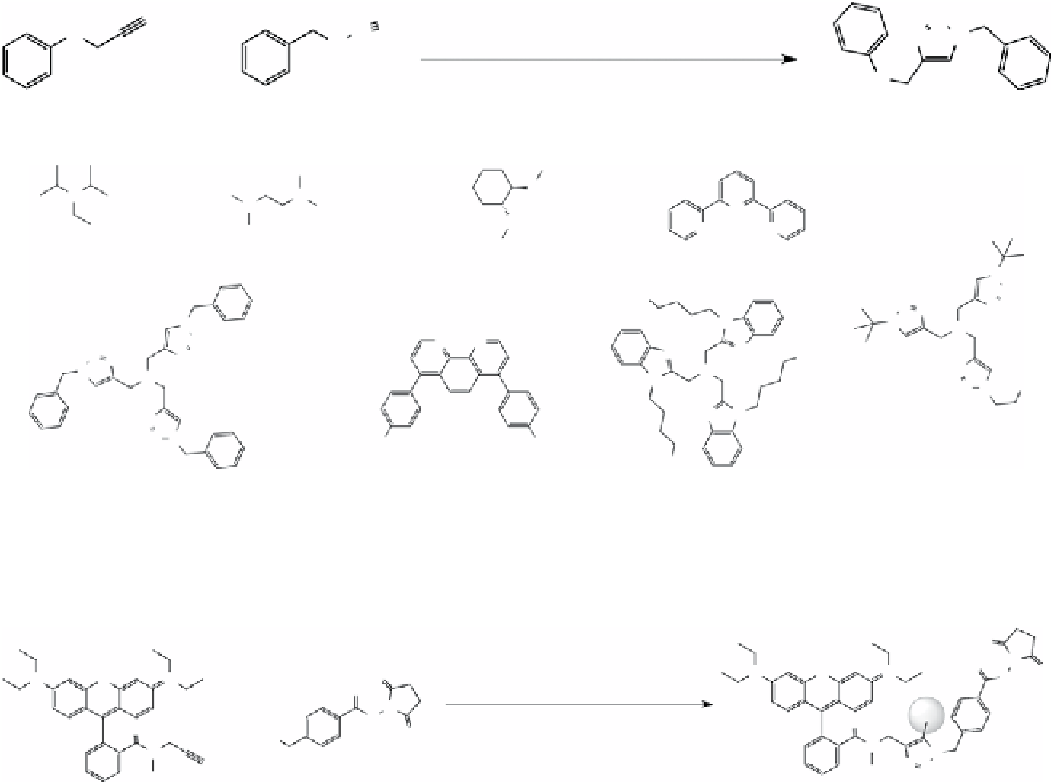
fIgure 2.3
Commonly used ligands in CuAAC.
O
-
-
Cl
N
Cl
+
O
+
O
N
O
N
O
O
N
O
N
CuCl
2
, Na
125
I, TEA, TEA, CH
3
CN
O
N
125
I
O
O
+
O
O
N
3
N
N
N
N
N
scheme 2.6
Incorporation of
125
I-label via CuAAC.
Addition of ligands (Figure 2.3), although not required in most cases, was found to accelerate the reaction with lowered
copper concentration, presumably by stabilising copper (I), protecting it from oxidation and disproportionation, and thereby
enhancing the catalytic activity of the copper (I) ion [63-67].
In the past a few years, the CuAAC has been studied and reviewed extensively, for its mechanistic analysis [68-70],
reactivity and general applications [71-76], application in small molecule synthesis [77, 78], polymer and material sciences
[79-83], synthesis of glycopolymers [20, 84], synthesis of peptidomimetics [85, 86], dendrimer construction [87, 88], prep-
aration of ligand-bound liposomes, modification of nucleic acids [89-92], modification of solid surfaces [93-96], modification
of polymeric nanoparticles [18, 97, 98], modification of carbon nanotubes [99], modification of virus particles [63, 100], and
bioconjugation of peptide or proteins [101, 102].
To prepare molecular imaging probes, CuAAC has been used to introduce F-18 to small molecule inhibitors of target
proteins [103-105], folates [106, 107], peptides or glycopeptides [108-114], proteins (via azido Fdg) [115], and oligonu-
cleotides [116-118], C-11[119] and Tc-99m can also be introduced to carrier molecules effortlessly [120-122].
Interestingly, in a recent report, I-125 was incorporated to the triazole ring during the cyclisation reaction when CuI was
used as a copper source, leading to an easy one-pot synthesis of multimodal imaging probe (Scheme 2.6) [123]. A few
MRI probes were also synthesised using CuAAC, which usually coupled chelators or ligands of contrast agents to a
carrier molecule, followed by the addition of contrast metal salts [124-126]. In a recent report, pre-labelled gd chelate
was successfully introduced to dendrimers using CuAAC [127]. Surface modification of iron oxide nanoparticles has
been very efficient with CuAAC as seen in the cases of introduction of biotin, fluorochrome, or steroid moieties [128],
targeting groups [109], cell-penetrating ligands [129, 130], and antibodies [131]. Similarly, grafting of Peg to self-
assembled polyelectrolyte nanoparticles was made promptly with CuAAC [132].
Most often, the CuAAC reaction has been performed with CuSo
4
/ascorbate in either water or water/organic solvents or with
CuI in organic solvents, such as THF, CH
3
Cn, dMF, dMSo, dCM, toluene, with or without ligands, at room temperature or