Chemistry Reference
In-Depth Information
Protein
Biotin
Biotin
N
Buffer
Protein-N
3
+
N
N
scheme 2.7
Strain-promoted azide-alkyne cycloaddition.
600
1000
500
800
400
600
300
400
200
200
100
0
0
OCT
DIMAC
MOFO
DIFO-2
DIFO-3
DIFO
DIBO*
BCN*
DIBAC**
BARAC
F
F
F
COOH MeO
F
F
O
F
MeO
N
N
HH
COOH
O
HO
COOH
O
COOH
OH
O
OH
N
COOH
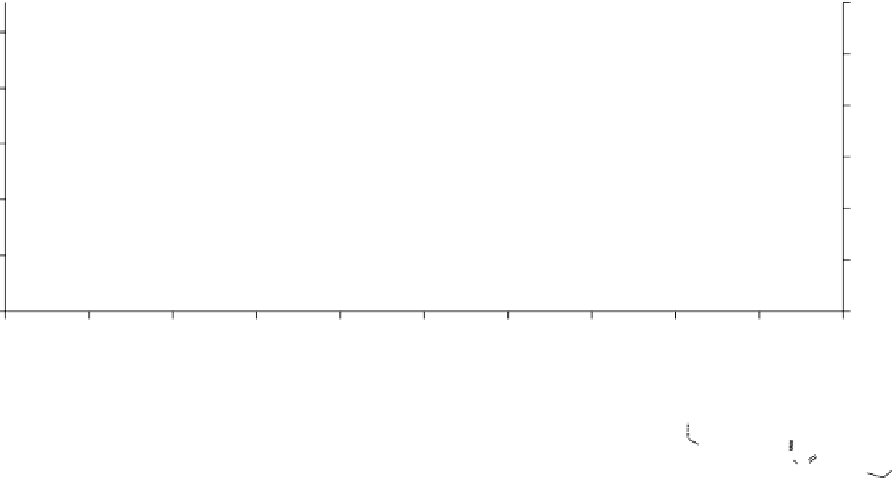
fIgure 2.5
Reactivity (second-order rate constant) and lipophilicity (logP) of various cyclooctynes. data was plotted from values in
references. Reactions were in acetonitrile, acetonitrile-water (*) and methanol (**).
reaction to give triazole as a single product [140], bertozzi and co-workers developed the 'copper-free' strain-promoted
azide-alkyne cycloaddition as a bioconjugation method for use under physiological conditions (Scheme 2.7) [141, 142].
Azido-proteins either in buffers or on a living cell surface could be labelled efficiently in 1 h under ambient conditions, and
the reaction was more efficient than Staudinger ligation (around two-fold).
To enhance the efficiency of the cyclooctyne-azide reaction, electron-withdrawing groups were introduced into cyclooc-
tyne, leading to their revolutionary cyclooctyne - difluorinated cyclooctyne (dIFo) (Figure 2.5) [143]. The combined effects
of ring-strain release and favourable electronic properties greatly accelerated the cycloocyne-azide cycloaddition to be
comparable with CuAAC in protein labelling. Its second-order rate constant was measured to be 7.6 × 10
-2
M
-1
s
-1
(with
benzyl azide), which is 17 to 63 times faster than the Staudinger ligation or previous strain-promoted cycloadditions.
Labelling of azido-glycan on the live cell surface was observed after 1 minute reaction with the dIFo fluorescent probe.
besides the highly sensitive azide labelling, dIFo-azide cycloaddition did not show cellular cytotoxicity as assessed by
morphology and propidium iodide staining. These features allowed the dynamic imaging of glycan internalisation and sub-
cellular partitioning in living cells, and its compatibility with glycan trafficking in the time examined was verified by two-
colour imaging using dIFo tethered fluorescent probes. In zebrafish embryos, the dIFo fluorescent probe was applied to
label the metabolically generated azido-glycans, fluorescence was observed after 1 minute reaction with dIFo probe, and
the intensity increased with reaction time [144]. using multicolour dIFo fluorescent probes during zebrafish embryogenesis,
spatiotemporal glycan expression was visualised to reveal its differences in the cell-surface expression, intracellular traffick-
ing, and tissue distribution in the embryo. The initial breakthrough in imaging glycans in zebrafish embryo by cyclooctyne-
azide cycloaddition led to successful studies of glycan expression and trafficking in living
Caenorhabditis elegans
[145], in
zebrafish during early embryogenesis [146, 147], and in live mice [148].
Although very efficient and biocompatible, the use of dIFo may be limited due to its time-consuming synthesis. The bertozzi
group reported a more tractable synthetic route with a 20-fold increase in overall yields, and the products were comparable to
the originally reported dIFo in terms of reaction efficiency and low cytotoxicity [150]. boons and co-workers reported the use