Chemistry Reference
In-Depth Information
2.2.2.1 Staudinger Ligation
The Staudinger reaction occurs rapidly between an azide and a triphenylphosphine to form
an aza-ylide, which hydrolyzes spontaneously to generate a primary amine (Figure 2.1a) [49]. bertozzi and co-workers
redesigned the molecule by introducing a well-positioned electrophilic trap in the phosphine structure, which could capture
the nucleophilic aza-ylide intermediate and form an amide bond upon hydrolysis (Figure 2.1b) [50]. The mild reaction
conditions allowed the introduction of a biotin molecule to living Jurkat cell surface, which was metabolically engineered
to present azido groups.
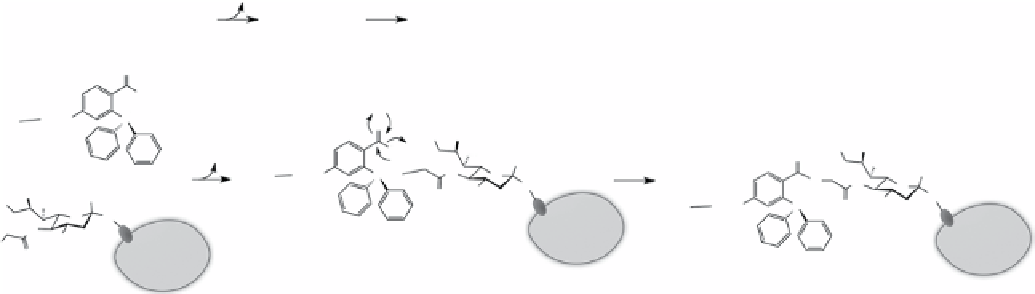
Following the original work on Staudinger ligation reactions, the bertozzi group reported studies on 'traceless' Staudinger
ligation to form an amide bond between the two components on the phosphine and azide side, without other additional atoms in
the linkage (Scheme 2.3) [51]. Although only preliminary work was done to show the formation of amide bonds, the work certainly
shone light on its application in peptide coupling and modification of cellular components such as proteins and glycans.
At about the same time, Raine and co-workers reported their successful coupling of two amino acids using a two-step
traceless Staudinger ligation (Scheme 2.4) [52]. The first step involved a trans-thioesterification of amino acid thioester with
phosphinothiol, and the second step resembled that used in bertozzi's “traceless” Staudinger ligation.
both nontraceless and traceless Staudinger ligations exhibit rapid kinetics and can be performed in living systems due to
abiotic nature of their reagents. The ligation reactions have found their widespread use in the modification of glycans, pro-
teins, lipids, and dnA molecules, in the context of small molecules, polymers, and solid supports. Past and recent examples
(a)
N
2
H
2
O
R
3
P
+
—N
-
-R
′
R
3
P: +N
≡
N
+
-N
-
-R
′
R
3
P=O +H
2
N-R
′
(b)
Aza-ylide
O
OCH
3
Electrophilic trap
P
biotin
linker
O
HO
OH
HO
OH
OCH
3
P
+
-N
-
OH
N
2
O
N
COOH
OH
H
2
O
N
COOH
+
biotin
linker
O
H
O
O
HO
HOOC
HO
O
O
HO
OH
HO
P=O
biotin
linker
O
O
O
HN
O
aza-ylide
HO
N
3
Formation of amide bond
fIgure 2.1
(a) Traditional Staudinger reaction. (b) Staudinger ligation used to label azide-containing cell surface glycans with biotin.
NHBz
NHBz
NHBz
N
Ph
N
Ph
N
N
O
-
N
N
N
2
Ph
2
P
P
N
H
2
O
N
N
+
O
O
N
N
N
N
N
N
3
+
O
O
Wet THF
half life=18 h
H
O
Ph
O
OH
Ph
OH
P
OH
O
O
OH
scheme 2.3
Traceless Staudinger ligation to form an amide bond.
SH
O
N
PPh
2
O
O
N
N
N
2
O
S
N
H
N
Ph
+
H
2
O
N
H
O
PPh
2
S
Ph
-
S
Ph
N
O
O
O
O
Ph
O
PPh
2
N
Ph
N
Ph
SH
O
Ph
N
3
O
PPh
2
O
scheme 2.4
Traceless Staudinger ligation to form amide bond between two amino acids.